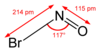
Nitrosyl bromide
Another to separate nitrosyl bromide into NO and Br or Br2 is by having excess of NO which then the experiment will follow first order kinetics.
This reverse rate constant was calculated to be kr = 2.29 ± 0.33 x 10-21 cm3 /molecules With excess Br2 plus NO the reaction follows third order kinetics.
Hippler studied the recombination of Br atoms after photoylsis of less than 0.3 torr of Br2 at room temperate in the range of 1 - 100 atm.
Godfrey examined the kinetics of BrNO formation and destruction using time resolved photolysis techniques.
He also included the effects of the loss of Br2 to internal surfaces of the cell in calculations of the reaction rate constants.