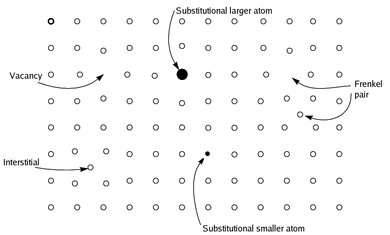
Non-stoichiometric compound
The type of equilibrium defects in non-stoichiometric compounds can vary with attendant variation in bulk properties of the material.
[1] Non-stoichiometric compounds also exhibit special electrical or chemical properties because of the defects; for example, when atoms are missing, electrons can move through the solid more rapidly.
[not verified in body] Non-stoichiometric compounds have applications in ceramic and superconductive material and in electrochemical (i.e., battery) system designs.
[2]: 642–644 For example, although wüstite (ferrous oxide) has an ideal (stoichiometric) formula FeO, the actual stoichiometry is closer to Fe0.95O.
[citation needed] Many useful compounds are produced by the reactions of hydrocarbons with oxygen, a conversion that is catalyzed by metal oxides.
The process operates via the transfer of "lattice" oxygen to the hydrocarbon substrate, a step that temporarily generates a vacancy (or defect).
[6] An analogous sequence of events describes other kinds of atom-transfer reactions including hydrogenation and hydrodesulfurization catalysed by solid catalysts.
For example, yttrium barium copper oxide, arguably the most notable high-temperature superconductor, is a non-stoichiometric solid with the formula YxBa2Cu3O7−x.
[6] It was mainly through the work of Nikolai Semenovich Kurnakov and his students that Berthollet's opposition to Proust's law was shown to have merit for many solid compounds.
Kurnakov divided non-stoichiometric compounds into berthollides and daltonides depending on whether their properties showed monotonic behavior with respect to composition or not.
[7] The names come from Claude Louis Berthollet and John Dalton, respectively, who in the 19th century advocated rival theories of the composition of substances.