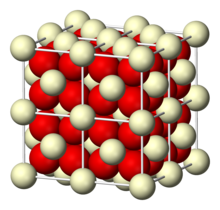
Cerium(IV) oxide
After extraction of the metal ions into aqueous base, Ce is separated from that mixture by addition of an oxidant followed by adjustment of the pH.
These states form a wide dispersive band, extending over a region of some eV, which can be correctly detected using theoretical methods accurately.
At high temperatures it releases oxygen to give a non-stoichiometric, anion deficient form that retains the fluorite lattice.
The equation has been shown to predict the equilibrium non-stoichiometry x over a wide range of oxygen partial pressures (103–10−4 Pa) and temperatures (1000–1900 °C).
The loss of oxygen continues into the molten liquid state where the local Ce-O coordination number drops to predominantly 6-fold, compared to 8-fold in the stoichiometric fluorite structure.
[10] In the most stable fluorite phase of ceria, it exhibits several defects depending on partial pressure of oxygen or stress state of the material.
Undoped and doped ceria also exhibit high electronic conductivity at low partial pressures of oxygen due to reduction of the cerium ion leading to the formation of small polarons.
One small but illustrative use is its use in the walls of self-cleaning ovens as a hydrocarbon oxidation catalyst during the high-temperature cleaning process.
[26] Building on its distinct surface interactions, ceria finds further use as a sensor in catalytic converters in automotive applications, controlling the air-exhaust ratio to reduce NOx and carbon monoxide emissions.
[32] It effectively removes minor scratches and imperfections from glass surfaces through both mechanical abrasion and chemical interaction, producing a smooth, high-gloss finish.
[33] Cerium oxide can also enhance the durability of optical surfaces by forming a protective layer that increases resistance to scratches and environmental wear.
[36][37][38] Nanoceria is a prospective replacement of zinc oxide and titanium dioxide in sunscreens, as it has lower photocatalytic activity.