Nuclear reaction
Various nuclear fusion reactions of light elements power the energy production of the Sun and stars.
The feat was popularly known as "splitting the atom", although it was not the modern nuclear fission reaction later (in 1938) discovered in heavy elements by the German scientists Otto Hahn, Lise Meitner, and Fritz Strassmann.
[1] Nuclear reactions may be shown in a form similar to chemical equations, for which invariant mass must balance for each side of the equation, and in which transformations of particles must follow certain conservation laws, such as conservation of charge and baryon number (total atomic mass number).
(The He-4 nucleus is unusually stable and tightly bound for the same reason that the helium atom is inert: each pair of protons and neutrons in He-4 occupies a filled 1s nuclear orbital in the same way that the pair of electrons in the helium atom occupy a filled 1s electron orbital).
The energy released in a nuclear reaction can appear mainly in one of three ways: When the product nucleus is metastable, this is indicated by placing an asterisk ("*") next to its atomic number.
An example of a large repository of reaction rates is the REACLIB database, as maintained by the Joint Institute for Nuclear Astrophysics.
In the initial collision which begins the reaction, the particles must approach closely enough so that the short-range strong force can affect them.
As most common nuclear particles are positively charged, this means they must overcome considerable electrostatic repulsion before the reaction can begin.
Thus, such particles must be first accelerated to high energy, for example by: Also, since the force of repulsion is proportional to the product of the two charges, reactions between heavy nuclei are rarer, and require higher initiating energy, than those between a heavy and light nucleus; while reactions between two light nuclei are the most common ones.
In fact, at extremely low particle energies (corresponding, say, to thermal equilibrium at room temperature), the neutron's de Broglie wavelength is greatly increased, possibly greatly increasing its capture cross-section, at energies close to resonances of the nuclei involved.
These are particularly useful in experimental nuclear physics, because the reaction mechanisms are often simple enough to calculate with sufficient accuracy to probe the structure of the target nucleus.
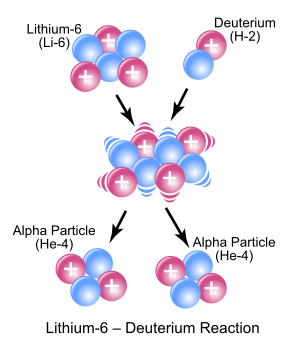
3 Li
) and deuterium ( 2
1 H
) react to form the highly excited intermediate nucleus 8
4 Be
which then decays immediately into two alpha particles of helium-4 ( 4
2 He
). Protons are symbolically represented by red spheres, and neutrons by blue spheres.