O-linked glycosylation
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein.
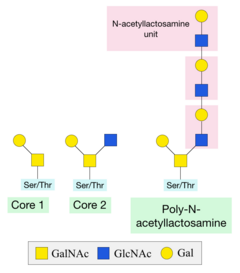
[8] Mucins are a group of heavily O-glycosylated proteins that line the gastrointestinal and respiratory tracts to protect these regions from infection.
O-GlcNAcylation and phosphorylation can occur on the same threonine and serine residues, suggesting a complex relationship between these modifications that can affect many functions of the cell.
[14][13] Additionally, O-GlcNAcylation can enhance the Warburg Effect, which is defined as the change that occurs in the metabolism of cancer cells to favour their growth.
[6][15] Because both O-GlcNAcylation and phosphorylation can affect specific residues and therefore both have important functions in regulating signalling pathways, both of these processes provide interesting targets for cancer therapy.
O-mannosylation involves the transfer of a mannose from a dolichol-P-mannose donor molecule onto the serine or threonine residue of a protein.
[16] Until recently, it was believed that the process is restricted to fungi, however it occurs in all domains of life; eukaryotes, (eu)bacteria and archae(bacteri)a.
[16] O-Man sugars separate two domains of the protein, required to connect the extracellular and intracellular regions to anchor the cell in position.
[18] Ribitol, xylose and glucuronic acid can be added to this structure in a complex modification that forms a long sugar chain.
Without these modifications, the glycoprotein cannot anchor the cell which leads to congenital muscular dystrophy (CMD), characterised by severe brain malformations.
[16] O-galactose is commonly found on lysine residues in collagen, which often have a hydroxyl group added to form hydroxylysine.
Addition of a galactose to the hydroxyl group is initiated in the endoplasmic reticulum, but occurs predominantly in the Golgi apparatus and only on hydroxylysine residues in a specific sequence.
[7] Addition of fucose sugars to serine and threonine residues is an unusual form of O-glycosylation that occurs in the endoplasmic reticulum and is catalysed by two fucosyltransferases.
[1] Similarly to O-fucosylation, O-glucosylation is an unusual O-linked modification as it occurs in the endoplasmic reticulum, catalysed by O-glucosyltransferases, and also requires a defined sequence in order to be added to the protein.
[25] Proteoglycans consist of a protein with one or more sugar side chains, known as glycosaminoglycans (GAGs), attached to the oxygen of serine and threonine residues.
Proteoglycans are usually found on the cell surface and in the extracellular matrix (ECM), and are important for the strength and flexibility of cartilage and tendons.
[26] Different types of proteoglycans exist, depending on the sugar that is linked to the oxygen atom of the residue in the protein.
[6] Keratan sulphate attaches to a serine or threonine residue through GalNAc, and is extended with two galactose sugars, followed by repeating units of glucuronic acid (GlcA) and GlcNAc.
[26] Galactose or glucose sugars can be attached to a hydroxyl group of ceramide lipids in a different form of O-glycosylation, as it does not occur on proteins.
Lewis epitopes are important in determining blood groups, and allow the generation of an immune response if we detect foreign organs.
O-glycan structures, and especially the terminal Lewis epitopes, are important in allowing tumor cells to invade new tissues during metastasis.