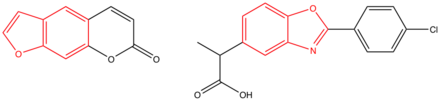
Benoxaprofen
It is a non-steroidal anti-inflammatory drug (NSAID) of the arylpropionic acid class, and was marketed under the brand name Opren in the United Kingdom and Europe by Eli Lilly and Company (commonly referred to as Lilly), and as Oraflex in the United States of America (USA).
[1] Lilly began Phase I of the benoxaprofen clinical trials by testing a selection of healthy human volunteers.
[1] When Lilly formally requested to begin marketing benoxaprofen in January 1980 with the US FDA, the document consisted of more than 100,000 pages of test results and patients records.
The British Medical Journal reported in May 1982 that physicians in the United Kingdom believed that the drug was responsible for at least twelve deaths, mainly caused by kidney and liver failure.
The British Committee on the Safety of Medicines declared, in a telegram to the FDA, that it had received reports of more than 3,500 adverse side effects among patients who had used Oraflex.
Almost simultaneously, the FDA said it had reports of 11 deaths in the USA among Oraflex users, most of which were caused by kidney and liver damage.
This is due to the co-planarity of the benzoxazole and phenyl rings, but the molecule also has a non-planar side chain consisting of the propanoic acid moiety which acts as a carrier group.
Irradiation of benoxaprofen in an aqueous solution causes photochemical decarboxylation via a radical mechanism and in single-strand breaks of DNA.
The plasma levels of benoxaprofen in eleven subjects have been accurately predicted, based on the two-compartment open model.
[6] In female rats, after oral dose of 20 mg/kg, the tissue concentration of benoxaprofen was the highest in liver, kidney, lungs, adrenals, and ovaries.
[11] A study shows that benoxaprofen, or other lipoxygenase-inhibiting agents, might be helpful in the treatment of psoriasis because the migration inhibition of the inflammatory cells (leukocytes) into the skin.
[12] Gastrointestinal side effects of benoxaprofen are bleeding, diarrhoea, abdominal pain, anorexia, mouth ulcers, and taste change.
[4] Benoxaprofen has a rather long half-life in man (t1/2= 20-30 hours), undergoes biliary excretion and enterohepatic circulation, and is also known to have a slow plasma clearance (CL p=4.5 millilitre per minute).
[4] The fetal hepatotoxicity of benoxaprofen can be attributed to the accumulation of the drug after a repeated dosage, and also associated with the slow plasma clearance.
The toxic metabolites may bind to vital intracellular macromolecules, and may generate reactive oxygens by redox cycling if quinone is formed.
[16] The observed skin phototoxicity of patients treated with benoxaprofen can be explained with a look at the structure of the compound.
Furthermore, possible explanations for the photochemical decarboxylation and oxygen radical formation may be the accumulation of repeated dosage, the induction of cytochrome P450I, and the emergence of reactive intermediates with covalent binding.
[7] In all six animals tested, which included rats, dogs, rhesus monkeys, rabbits, guinea pigs, and mice, the drug was well absorbed orally.
[7] The plasma half-life was found to be different, being less than 13 hours in the dog, rabbit, and monkey, it was notable longer in mice.
[7] Another research in rats showed that the plasma membrane of hepatocytes begun to form blebs after administration of benoxaprofen.
[7] A Sandmeyer reaction by diazotisation of 2-(4-aminophenyl)propanenitrile (1) followed by acid hydrolysis leads to the phenol (2), which is nitrated and reduced by catalytic hydrogenation to give the aminophenol (3).