Organic chemistry
His discovery, made widely known through its financial success, greatly increased interest in organic chemistry.
[9] A crucial breakthrough for organic chemistry was the concept of chemical structure, developed independently in 1858 by both Friedrich August Kekulé and Archibald Scott Couper.
[11] The era of the pharmaceutical industry began in the last decade of the 19th century when the German company, Bayer, first manufactured acetylsalicylic acid—more commonly known as aspirin.
[12] By 1910 Paul Ehrlich and his laboratory group began developing arsenic-based arsphenamine, (Salvarsan), as the first effective medicinal treatment of syphilis, and thereby initiated the medical practice of chemotherapy.
[13][14] His laboratory made decisive contributions to developing antiserum for diphtheria and standardizing therapeutic serums.
[15] Early examples of organic reactions and applications were often found because of a combination of luck and preparation for unexpected observations.
For example, cholesterol-related compounds have opened ways to synthesize complex human hormones and their modified derivatives.
Converting individual petroleum compounds into types of compounds by various chemical processes led to organic reactions enabling a broad range of industrial and commercial products including, among (many) others: plastics, synthetic rubber, organic adhesives, and various property-modifying petroleum additives and catalysts.
Research in the field increased throughout the twentieth century, without any indication of slackening in the rate of increase, as may be verified by inspection of abstraction and indexing services such as BIOSIS Previews and Biological Abstracts, which began in the 1920s as a single annual volume, but has grown so drastically that by the end of the 20th century it was only available to the everyday user as an online electronic database.
The physical properties of organic compounds typically of interest include both quantitative and qualitative features.
A well-known example of a sublimable organic compound is para-dichlorobenzene, the odiferous constituent of modern mothballs.
Neutral organic compounds tend to be hydrophobic; that is, they are less soluble in water than inorganic solvents.
Exceptions include organic compounds that contain ionizable groups as well as low molecular weight alcohols, amines, and carboxylic acids where hydrogen bonding occurs.
Various specialized properties of molecular crystals and organic polymers with conjugated systems are of interest depending on applications, e.g. thermo-mechanical and electro-mechanical such as piezoelectricity, electrical conductivity (see conductive polymers and organic semiconductors), and electro-optical (e.g. non-linear optics) properties.
By 1880 an explosion in the number of chemical compounds being discovered occurred assisted by new synthetic and analytical techniques.
[21] The concept of functional groups is central in organic chemistry, both as a means to classify structures and for predicting properties.
Dipole distance (measured in Angstroms) and steric hindrance towards the functional group have an intermolecular and intramolecular effect on the surrounding environment and pH level.
The heteroatom of heterocyclic molecules is generally oxygen, sulfur, or nitrogen, with the latter being particularly common in biochemical systems.
Additionally, they are prevalent in a wide range of biochemical compounds such as alkaloids, vitamins, steroids, and nucleic acids (e.g. DNA, RNA).
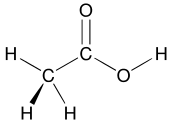
One important property of carbon is that it readily forms chains, or networks, that are linked by carbon-carbon (carbon-to-carbon) bonds.
Some are long-chain biopolymers, and these include peptides, DNA, RNA and the polysaccharides such as starches in animals and celluloses in plants.
Isoprenes in animals form the important steroid structural (cholesterol) and steroid hormone compounds; and in plants form terpenes, terpenoids, some alkaloids, and a class of hydrocarbons called biopolymer polyisoprenoids present in the latex of various species of plants, which is the basis for making rubber.
Biologists usually classify the above-mentioned biomolecules into four main groups, i.e., proteins, lipids, carbohydrates, and nucleic acids.
Petroleum and its derivatives are considered organic molecules, which is consistent with the fact that this oil comes from the fossilization of living beings, i.e., biomolecules.
Using a laser to vaporize graphite rods in an atmosphere of helium gas, these chemists and their assistants obtained cagelike molecules composed of 60 carbon atoms (C60) joined by single and double bonds to form a hollow sphere with 12 pentagonal and 20 hexagonal faces—a design that resembles a football, or soccer ball.
The C60 molecule was named buckminsterfullerene (or, more simply, the buckyball) after the American architect R. Buckminster Fuller, whose geodesic dome is constructed on the same structural principles.
The general theory of these reactions involves careful analysis of such properties as the electron affinity of key atoms, bond strengths and steric hindrance.
Synthetic organic chemistry is an applied science as it borders engineering, the "design, analysis, and/or construction of works for practical purposes".
For example, a carbonyl compound can be used as a nucleophile by converting it into an enolate, or as an electrophile; the combination of the two is called the aldol reaction.
The pieces, or the proposed precursors, receive the same treatment, until available and ideally inexpensive starting materials are reached.