Ethinylestradiol
[7][12] The general side effects of EE include breast tenderness and enlargement, headache, fluid retention, and nausea among others.
[7] Compared to estradiol, EE is more resistant to metabolism, has greatly improved bioavailability when taken by mouth, and shows relatively increased effects in certain parts of the body like the liver and uterus.
[7] These differences make EE more favorable for use in birth control pills than estradiol, though also result in an increased risk of blood clots and certain other rare adverse effects.
[20] The main reason for using HRT in menopausal women is to relieve common vasomotor symptoms such as hot flashes, night sweats, and flushing.
[12] In addition, there is a single preparation (brand name FemHRT) containing very low doses of EE (2.5 and 5 μg) plus a progestin in an oral tablet that remains in use for menopausal hormone therapy.
[42] General side effects of EE are the same as for other estrogens and include breast tenderness, headache, fluid retention (bloating), nausea, dizziness, and weight gain.
[47] It has been estimated that at least 300 to 400 healthy young women die each year in the United States due to VTE caused by EE-containing birth control pills.
[57][58][59] SHBG levels with birth control pills containing different progestins are increased by 1.5 to 2-fold with levonorgestrel, 2.5- to 4-fold with desogestrel and gestodene, 3.5- to 4-fold with drospirenone and dienogest, and 4- to 5-fold with cyproterone acetate.
[64] When used orally at high dosages, for instance as a form of high-dose estrogen therapy in men with prostate cancer and in women with breast cancer, synthetic and non-bioidentical estrogens like EE and diethylstilbestrol are associated with fairly high rates of severe cardiovascular complications such as VTE, myocardial infarction, and stroke.
[8] However, both EE and diethylstilbestrol nonetheless have highly disproportionate effects on liver protein synthesis, which is thought to be responsible for their cardiovascular toxicity.
[7][67] In contrast to oral synthetic estrogens like EE and diethylstilbestrol, high-dosage polyestradiol phosphate and transdermal estradiol have not been found to increase the risk of cardiovascular mortality or thromboembolism in men with prostate cancer.
[8] Because of its disproportionate effects on liver protein synthesis and associated cardiovascular risks, synthetic estrogens like EE and diethylstilbestrol are no longer used in menopausal hormone therapy.
[67] At the lower dosages that are now used in birth control pills, EE has been associated rarely with cholestatic hepatotoxicity similarly to 17α-alkylated androgens/anabolic steroids and 17α-ethynylated 19-nortestosterone progestins.
[74][72] With birth control pills containing 50 μg/day EE, alanine aminotransferase (ALT) levels increase by 50%, hematocrit by 19%, and leukocytes by 50%, while gamma-glutamyltransferase (GGT) decreases by 30%.
[77][78] At one time, EE-containing birth control pills were estimated to be responsible for 84% of all drug-related and histologically verified liver damage.
[72][76] The high doses of EE that were used in early COCs were associated with a significantly increased risk of endometrial cancer in certain preparations, for instance those containing the progestogen dimethisterone.
Chronic exposure to low levels of EE over seven years led to the collapse of fathead minnow populations in an experimental lake in Ontario, Canada.
[38] Examples of inducers include anticonvulsants like phenytoin, primidone, ethosuximide, phenobarbital, and carbamazepine; azole antifungals like fluconazole; and rifamycin antibiotics like rifampin (rifampicin).
[84] Examples of known interactions include bupropion, caffeine, mephenytoin, midazolam, nicotine, nifedipine, omeprazole, propranolol, proguanil, selegiline, theophylline, and tizanidine.
[97][98] In contrast, the potencies of EE and natural estrogens are similar when they are administered intravenously, due to the bypassing of first-pass metabolism.
[119][120][27][121] Birth control pills that contain EE are useful in the treatment of androgen-dependent conditions like acne and hirsutism by virtue of their antiandrogenic effects.
[7][10][125] Estrogens are antigonadotropins and are able to suppress the secretion of LH and FSH from the pituitary gland and by extension gonadal testosterone production.
[8] A stronger suppression of testosterone levels was observed in men following daily treatment with a combined oral contraceptive containing 50 μg ethinylestradiol and 0.5 mg norgestrel for 9 days.
[8] A combination of 20 μg/day EE and 10 mg/day methyltestosterone was found to suppress FSH secretion in men to an extent sufficient to stop spermatogenesis.
[98] In addition to its antigonadotropic effects, EE can significantly suppress androgen production by the adrenal glands at high concentrations.
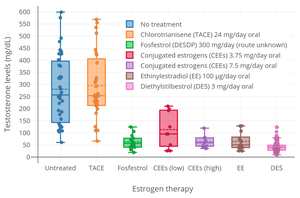
[8][139][140] One study found that treatment with a high dosage of 100 μg/day EE suppressed circulating adrenal androgen levels by 27 to 48% in transgender women.
[7] The reduced vaginal bleeding and spotting with EE is one of the main reasons that it is used in COCs instead of estradiol,[3] in spite of its potentially inferior safety profile (related to its adverse effects on hepatic protein synthesis and VTE incidence).
EE was the first orally active synthetic estrogen and was described in 1938 by Hans Herloff Inhoffen and Walter Hohlweg of Schering AG in Berlin.
[162][152] The generic name of EE in French and its DCFTooltip Dénomination Commune Française are éthinylestradiol, in Spanish is etinilestradiol, in Italian and its DCITTooltip Denominazione Comune Italiana are etinilestradiolo, and in Latin is ethinylestradiolum.
EE has been marketed as a standalone oral drug under the brand names Esteed, Estinyl, Feminone, Lynoral, Menolyn, Novestrol, Palonyl, Spanestrin, and Ylestrol among others, although most or all of these formulations are now discontinued.