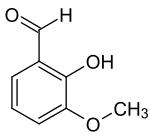
ortho-Vanillin
The "ortho-" prefix refers to the position of the compound’s hydroxyl moiety, which is found in the para-position in vanillin.
[4] By 1910, methods for its purification had been developed by Francis Noelting, who similarly demonstrated its versatility as a general synthetic precursor for a diverse array of compounds, such as the coumarins.
[6] ortho-Vanillin is harmful if ingested, irritating to eyes, skin and respiratory system, but has an unmistakable high LD50 of 1330 mg/kg in mice.
[7] It is a weak inhibitor of tyrosinase,[8] and displays both antimutagenic and comutagenic properties in Escherichia coli.
[11] Today, most ortho-vanillin is used in the study of mutagenesis and as a synthetic precursor for pharmaceuticals, for example, benafentrine[12] and an antiandrogen compound called Pentomone.