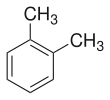
o-Xylene
o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2, with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration).
o-Xylene is a colourless slightly oily flammable liquid.
Most o-xylene is produced by cracking petroleum, which affords a distribution of aromatic compounds, including xylene isomers.
Net production was approximately 500,000 tons in the year 2000. o-Xylene is largely used in the production of phthalic anhydride, which is a precursor to many materials, drugs, and other chemicals.
[7] Related to their easy oxidation, the methyl groups are susceptible to halogenation.