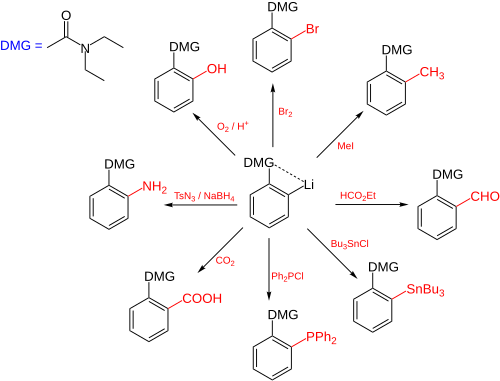
Directed ortho metalation
An aromatic ring system with a DMG group 1 interacts with an alkyllithium such as n-butyllithium in its specific aggregation state (hence (R-Li)n) to intermediate 2 since the hetero atom on the DMG is a Lewis base and lithium the Lewis acid.
The very basic alkyllithium then deprotonates the ring in the nearest ortho- position forming the aryllithium 3 all the while maintaining the acid-base interaction.
[5][6] The method has also been applied to the synthesis of enantiopure benzyl amines[7] in scheme 3,[8] which involves ortho-lithiation of tert-butyl phenyl sulfoxide.
On approach to the lithium intermediate, the bulky tosyl group on the imine electrophile is responsible for the asymmetric induction taking place.
In another application[9] DOM is applied in placing a bulky tert-butyl group in an ortho position (scheme 4).