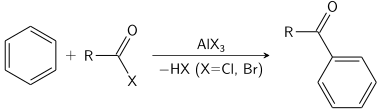Electrophilic aromatic substitution
Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates.
The overall reaction mechanism, denoted by the Hughes–Ingold mechanistic symbol SEAr,[3] begins with the aromatic ring attacking the electrophile E+ (2a).
This step leads to the formation of a positively charged and delocalized cyclohexadienyl cation, also known as an arenium ion, Wheland intermediate, or arene σ-complex (2b).
Many examples of this carbocation have been characterized, but under normal operating conditions these highly acidic species will donate the proton attached to the sp3 carbon to the solvent (or any other weak base) to reestablish aromaticity.
The capacity of these types of substituents to leave is sometimes exploited synthetically, particularly the case of replacement of silyl by another functional group (ipso attack).
Substituents can generally be divided into two classes regarding electrophilic substitution: activating and deactivating towards the aromatic ring.
On the other hand, deactivating substituents destabilize the intermediate cation and thus decrease the reaction rate by either inductive or resonance effects.
The deactivation of the aromatic system means that generally harsher conditions are required to drive the reaction to completion.
Halogens are electronegative, so they are deactivating by induction, but they have lone pairs, so they are resonance donors and therefore ortho/para directors.
Such activating groups donate those unshared electrons to the pi system, creating a negative charge on the ortho and para positions.
In addition to the increased nucleophilic nature of the original ring, when the electrophile attacks the ortho and para positions of aniline, the nitrogen atom can donate electron density to the pi system (forming an iminium ion), giving four resonance structures (as opposed to three in the basic reaction).
When the electrophile attacks the meta position, the nitrogen atom cannot donate electron density to the pi system, giving only three resonance contributors.
[4] Compared to benzene, the rate of electrophilic substitution on pyridine is much slower, due to the higher electronegativity of the nitrogen atom.
Additionally, the nitrogen in pyridine easily gets a positive charge either by protonation (from nitration or sulfonation) or Lewis acids (such as AlCl3) used to catalyze the reaction.
These compounds all contain an atom with an unshared pair of electrons (oxygen, sulfur, or nitrogen) as a member of the aromatic ring, which substantially stabilizes the cationic intermediate.
An early example concerns the addition of chloral to phenols catalyzed by aluminium chloride modified with (–)-menthol.