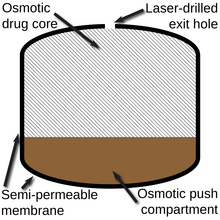
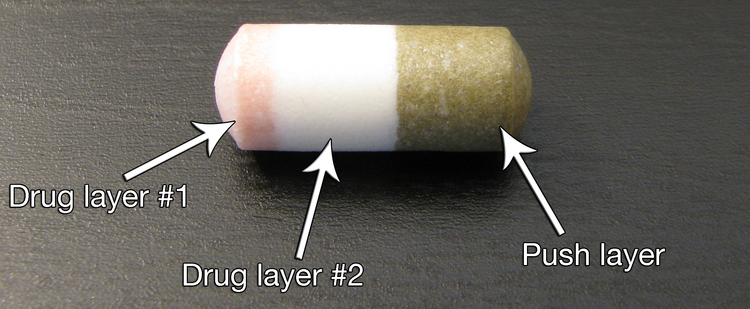
Osmotic-controlled release oral delivery system
[1] Merck & Co. later developed the Controlled-Porosity Osmotic Pump (CPOP) with the intention of addressing some of the issues that led to Osmosin's withdrawal via a new approach to the final stage of the release mechanism.
[1][2] In the early 1990s, an ALZA-funded research program began to develop a new dosage form of methylphenidate for the treatment of children with attention deficit hyperactivity disorder (ADHD).
[14] Methylphenidate's short half-life required multiple doses to be administered each day to attain long-lasting coverage, which made it an ideal candidate for the OROS technology.
Multiple candidate pharmacokinetic profiles were evaluated and tested in an attempt to determine the optimal way to deliver the drug, which was especially important given the puzzling failure of an existing extended-release formulation of methylphenidate (Ritalin SR) to act as expected.
The zero-order (flat) release profile that the PPOP was optimal at delivering failed to maintain its efficacy over time, which suggested that acute tolerance to methylphenidate formed over the course of the day.