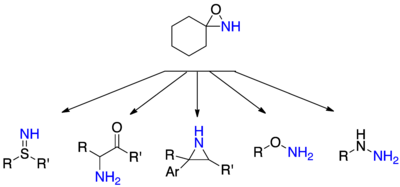
Oxaziridine
Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions.
[4] Whereas oxygen and nitrogen typically act as nucleophiles due to their high electronegativity, oxaziridines allow for electrophilic transfer of both heteroatoms.
This unusual reactivity is due to the presence of the highly strained three membered ring and the relatively weak N-O bond.
The unusual electronics of the oxaziridine system may be exploited to perform a number of oxygen and nitrogen transfer reactions including, but not limited to: α-hydroxylation of enolates, epoxidation of alkenes, selective oxidation of sulfides and selenides, amination of N-nucleophiles and N-acylamidation.
Some oxaziridines have the unique property of configurationally stable nitrogen atoms at room temperature due to an inversion barrier of 100 to 130 kJ/mol.
[7] In the late 1970s and early 1980s Franklin A. Davis synthesized the first N-sulfonyloxaziridines, which act exclusively as oxygen transfer reagents, and are the most predominantly used class of oxaziridines today.
[8] While originally synthesized with mCPBA and the phase transfer catalyst benzyltrimethylammonium chloride, an improved synthesis using oxone as the oxidant is now most prevalent.
Many millions of kilograms of hydrazine are produced annually by this method that involves a step wherein ammonia is oxidized in the presence of methyl ethyl ketone to give the oxaziridine:[20] In subsequent steps the oxaziridine is converted to the hydrazone, which is the immediate in the way to hydrazine: α-Hydroxyketones, or acyloins, are an important synthetic motifs present in many natural products.
α-Hydroxyketones have been synthesized in many ways, including reduction of α-diketones, substitution of a hydroxyl for a leaving group and direct oxidation of an enolate.
[10] Extensive work has been reported on asymmetric hydroxylation of prochiral enolates with camphorsulfonyloxaziridine derivatives, achieving moderate to high enantiomeric excess.
[16] In these instances it is proposed that the reaction proceeds through a closed transition state where the metal oxyanion is stabilized by chelation from the sulfate and coordinating groups on the camphor skeleton.
[27] Oxaziridines have been found to undergo rearrangement reactions via a radical mechanism when irradiated with UV light or in the presence of a single electron transfer reagent such as CuI.
[29] In light of this effect, it is possible to take advantage of the chiral nitrogen due to high inversion barrier to direct the rearrangement.
[28] Aubé takes advantage of this rearrangement as the key step in his synthesis of (+)-yohimbine,[28] a natural medicine classified by the NIH as possibly effective in the treatment of erectile dysfunction and the sexual problems caused by selective serotonin reuptake inhibitors.
[7] Oxaziridines undergo cycloaddition reactions with heterocumulenes to afford a number of unique five membered heterocycles, as shown in the figure below.