Nitrone
In organic chemistry, a nitrone is a functional group consisting of an N-oxide of an imine.
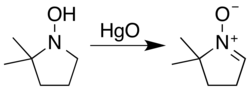
Secondary hydroxylamines oxidize to nitrones in air over a timescale of several weeks, a process cupric salts accelerate.
[1]: 477 In principle, N-alkylation could produce nitrones from oximes, but in practice electrophiles typically perform a mixture of N- and O-attack.
Like many other unsaturated functional groups, nitrones activate the α and β carbons towards reaction.
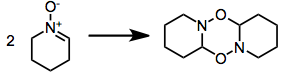
[6] For example, a dipolarophilic alkene combines to form isoxazolidine: Other ring-closing reactions are known,[7] including formal [3+3] and [5+2] cycloadditions.