Oxonium ion
For example, triethyloxonium tetrafluoroborate (Et3O+)(BF−4), a white crystalline solid, can be used, for example, to produce ethyl esters when the conditions of traditional Fischer esterification are unsuitable.
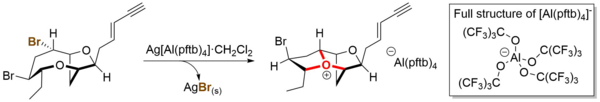
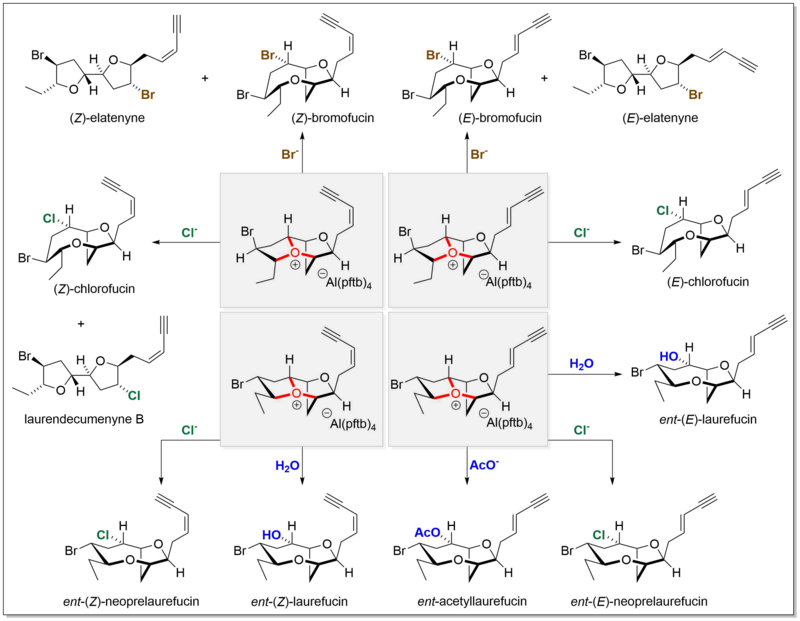
[10] Complex bicyclic and tricyclic oxonium ions have been proposed as key intermediates in the biosynthesis of a series of natural products by the red algae of the genus Laurencia.
[11] Several members of these elusive species have been prepared explicitly by total synthesis, demonstrating the possibility of their existence.
The resulting oxonium ions were characterized comprehensively by nuclear magnetic resonance spectroscopy at low temperature (−78 °C) with support from density functional theory computation.
These oxonium ions were also demonstrated to directly give rise to multiple related natural products by reacting with various nucleophiles, such as water, bromide, chloride, and acetate.