Ozonolysis
The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.
If ozonolysis is performed by introducing a stream of ozone-enriched oxygen through the reaction mixture, the effluent gas can be directed through a potassium iodide solution.
[7] Otherwise, the presence of unreacted ozone in solution (seeing its blue color) or in the bubbles (via iodide detection) only indicates when all alkenes have reacted.
The oxide and aldehyde or ketone react again in a 1,3-dipolar cycloaddition, producing a relatively stable ozonide intermediate, known as a trioxolane (4).
When 17O-labelled benzaldehyde reacts with carbonyl oxides, the label ends up exclusively in the ether linkage of the ozonide.
[16] Before the advent of modern spectroscopic techniques, the ozonolysis was an important method for determining the structure of organic molecules.
Chemists would ozonize an unknown alkene to yield smaller and more readily identifiable fragments.
Ozonolysis of alkynes generally gives an acid anhydride or diketone product,[17] not complete fragmentation as for alkenes.
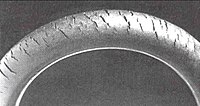
Ozonolysis produces surface ketone groups that can cause further gradual degradation via Norrish reactions if the polymer is exposed to light.
Ozone cracking was once commonly seen in the sidewalls of tires, where it could expand to cause a dangerous blowout, but is now rare owing to the use of modern antiozonants.
Other means of prevention include replacing susceptible rubbers with resistant elastomers such as polychloroprene, EPDM or Viton.