Buffer solution
[1] Its pH changes very little when a small amount of strong acid or base is added to it.
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
In nature, there are many living systems that use buffering for pH regulation.
Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle.
Because of this, the hydrogen ion concentration increases by less than the amount expected for the quantity of strong acid added.
Similarly, if strong alkali is added to the mixture, the hydrogen ion concentration decreases by less than the amount expected for the quantity of alkali added.
In Figure 1, the effect is illustrated by the simulated titration of a weak acid with pKa = 4.7.
The relative concentration of undissociated acid is shown in blue, and of its conjugate base in red.
Buffer capacity is a quantitative measure of the resistance to change of pH of a solution containing a buffering agent with respect to a change of acid or alkali concentration.
With either definition the buffer capacity for a weak acid HA with dissociation constant Ka can be expressed as[4][5][3]
Kw is the equilibrium constant for self-ionization of water, equal to 1.0×10−14.
Note that in solution H+ exists as the hydronium ion H3O+, and further aquation of the hydronium ion has negligible effect on the dissociation equilibrium, except at very high acid concentration.
This equation shows that there are three regions of raised buffer capacity (see figure 2).
In biological systems this is an essential condition for enzymes to function correctly.
For example, in human blood a mixture of carbonic acid (H2CO3) and bicarbonate (HCO−3) is present in the plasma fraction; this constitutes the major mechanism for maintaining the pH of blood between 7.35 and 7.45.
Outside this narrow range (7.40 ± 0.05 pH unit), acidosis and alkalosis metabolic conditions rapidly develop, ultimately leading to death if the correct buffering capacity is not rapidly restored.
If the pH value of a solution rises or falls too much, the effectiveness of an enzyme decreases in a process, known as denaturation, which is usually irreversible.
In industry, buffering agents are used in fermentation processes and in setting the correct conditions for dyes used in colouring fabrics.
For alkaline buffers, a strong base such as sodium hydroxide may be added.
Similarly, an alkaline buffer can be made from a mixture of the base and its conjugate acid.
By combining substances with pKa values differing by only two or less and adjusting the pH, a wide range of buffers can be obtained.
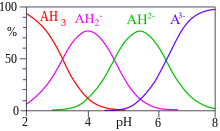
Citric acid is a useful component of a buffer mixture because it has three pKa values, separated by less than two.
First write down the equilibrium expression This shows that when the acid dissociates, equal amounts of hydrogen ion and anion are produced.
To find x, use the formula for the equilibrium constant in terms of concentrations:
Substitute the concentrations with the values found in the last row of the ICE table:
When the difference between successive pKa values is less than about 3, there is overlap between the pH range of existence of the species in equilibrium.
In the case of citric acid, this entails the solution of the two equations of mass balance:
CA is the analytical concentration of the acid, CH is the analytical concentration of added hydrogen ions, βq are the cumulative association constants.
The speciation diagram for citric acid was produced with the program HySS.
Cumulative association constants are used in general-purpose computer programs such as the one used to obtain the speciation diagram above.