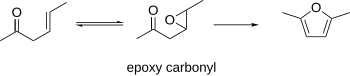
Paal–Knorr synthesis
It is a synthetically valuable method for obtaining substituted furans and pyrroles, which are common structural components of many natural products.
It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes.
The amine attacks the other carbonyl to form a 2,5-dihydroxytetrahydropyrrole derivative which undergoes dehydration to give the corresponding substituted pyrrole.
The initial diketone is converted to a thioketone with a sulfurizing agent, which then undergoes the same mechanism as the furan synthesis.
Synthesis of a thiophene requires a sulfurizing agent which is typically a sufficient dehydrator, such as phosphorus pentasulfide, Lawesson's reagent, or hydrogen sulfide.
The Paal–Knorr was also considered limited by harsh reaction conditions, such as prolonged heating in acid, which may degrade sensitive functionalities in many potential furan precursors.
Current methods allow for milder conditions that can avoid heat altogether, including microwave catalyzed cyclizations.
[11] The significance of this variation is in the fact that it increases the scope of the Paal–Knorr by taking advantage of the wealth of acetylene chemistry that exists, specifically that for the generation of propargyl alcohols.
Traditional Paal–Knorr conditions involved prolonged heating of strong acids to drive dehydration which occurred over a period of several hours.
Microwave-assisted Paal–Knorr reactions have been demonstrated to occur on time scales measured in minutes and in open flasks at room temperature.
[15] Heating the 1,4-diketone with ammonium acetate in methanol with camphor sulfonic acid and 4 angstrom molecular sieves gave the pyrrole with no N-substitution.