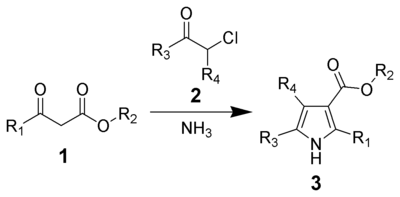
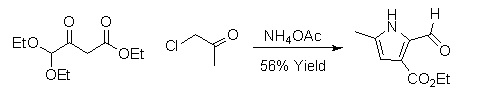
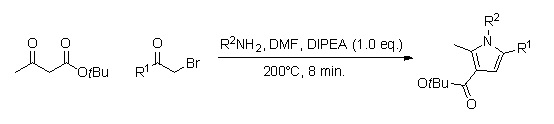
Hantzsch pyrrole synthesis
This reaction involves the high-speed vibration milling (HSVM) of ketones with N-iodosuccinimide (NIS) and p-toluenesulfonic acid, to form an α-iodoketone in situ.
This is followed by addition of a primary amine, a β-dicarbonyl compound, cerium(IV) ammonium nitrate (CAN) and silver nitrate, as shown in the scheme below: 2,3-dicarbonylated pyrroles can be synthesized by a version of the Hantzsch Pyrrole Synthesis.
The reaction can also occur between an enamine and an α-haloketone to synthesize substituted indoles, which also have biological significance.
[6][9] A library of substituted pyrrole analogs can be quickly produced by using continuous flow chemistry (reaction times of around 8 min.).
[10] The advantage of using this method, as opposed to the in-flask synthesis, is that this one does not require the work-up and purification of several intermediates, and could therefore lead to a higher percent yield.