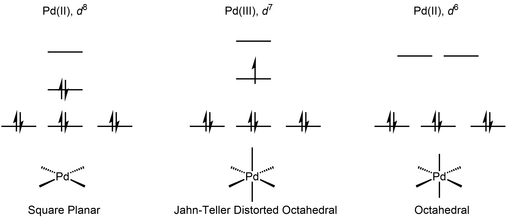
Palladium(III) compounds
X-ray crystallography revealed the expected Jahn–Teller distorted octahedral geometry, in spite of the highly symmetric structure of the ligand.
[4] Organopalladium complexes supported with a macrocyclic tetradentate ligand undergo single-electron oxidation to give Pd(III) species that is stabilized by the axially-positioned amine.
[9] Through X-ray crystallography, Ritter unambiguously showed that dinuclear Pd(III) complex is formed when the palladacycle is treated with two-electron oxidant, and such dinuclear complex undergoes C-Cl reductive elimination under ambient temperature.
Both experimental and computational data was consistent with a concerted 1,1-reductive elimination mechanism for the C-Cl forming step.
[10][11] The authors show that such bimetallic participation of redox event lowers the activation barrier for reductive elimination step by ~30 kcal/mol compared to a monometallic pathway.