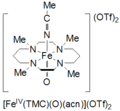High-valent iron
The synthetic compounds discussed below contain highly oxidized iron in general, as the concepts are closely related.
Oxoferryl species are commonly proposed as intermediates in catalytic cycles, especially biological systems in which O2 activation is required.
A second method for formation of cyclam oxoiron(IV) is reported as the reaction of FeII(TMC)(OTf)2 with 3 equivalents of H2O2 for 3 hours.
[6] High-valent iron bispidine complexes can oxidize cyclohexane to cyclohexanol and cyclohexanone in 35% yield with an alcohol to ketone ratio up to 4.
This deep green compound (two λmax at 445 and 630 nm respectively) is stable at 77 K. The stabilization of Fe(V) is attributed to the strong π–donor capacity of deprotonated amide nitrogens.
Although it is classified as a weak base, concentrated solutions of ferrate(VI) are only stable at high pH.
[13][14] A widely applicable method to generate high-valent nitridoiron species is the thermal or photochemical oxidative elimination of molecular nitrogen from an azide complex.
[15][16][17] The first nitridoiron(V) compound was synthesised and characterized by Wagner and Nakamoto (1988, 1989) using photolysis and Raman spectroscopy at low temperatures.