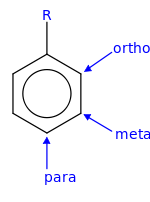
Arene substitution pattern
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.
Although the specifics vary depending on the compound, in simple disubstituted arenes, the three isomers tend to have rather similar boiling points.
Several methods exist in order to separate these isomers: The prefixes ortho, meta, and para are all derived from Greek, meaning correct, following, and beside, respectively.
[6][7] It was the German chemist Karl Gräbe who, in 1869, first used the prefixes ortho-, meta-, para- to denote specific relative locations of the substituents on a disubstituted aromatic ring (namely naphthalene).
For example, nicotinamide and niacin, shown meta substitutions on a pyridine ring, while the cation of pralidoxime is an ortho isomer.