Decay product
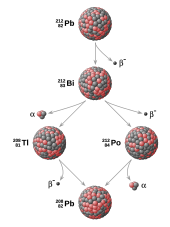
In many cases, individual members of the decay chain are as radioactive as the parent, but far smaller in volume/mass.
Thus, although uranium is not dangerously radioactive when pure, some pieces of naturally occurring pitchblende are quite dangerous owing to their radium-226 content,[2] which is soluble and not a ceramic like the parent.
Although it cannot be predicted whether any given atom of a radioactive substance will decay at any given time, the decay products of a radioactive substance are extremely predictable.
Such studies are done to measure pollution levels (in and around nuclear facilities) and for other matters.
This nuclear physics or atomic physics–related article is a stub.