Parvoviridae
The coding portion of the genome is flanked by telomeres at each end that form into hairpin loops that are important during replication.
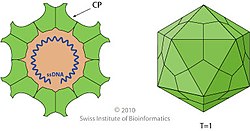
Parvovirus virions are small compared to most viruses, at 23–28 nanometers in diameter, and contain the genome enclosed in an icosahedral capsid that has a rugged surface.
Human parvoviruses are less severe, the two most notable being parvovirus B19, which causes a variety of illnesses including fifth disease in children, and human bocavirus 1, which is a common cause of acute respiratory tract illness, especially in young children.
They take their name from the Latin word parvum, meaning 'small' or 'tiny', referring to the small size of the virus's virions.
Most parvoviruses contain a transcriptional activation domain near the C-terminus that upregulates transcription from viral promoters as well as alternate or overlapping open reading frames that encode a small number of supporting proteins involved in different aspects of the viral life cycle.
[2] The coding portion of the genome is flanked at each end by terminal sequences about 116–550 nucleotides (nt) in length that consist of imperfect palindromes folded into hairpin loop structures.
When the genome is converted to double-stranded forms, replication origin sites are created involving sequences in and adjacent to the hairpins.
[2][4][5] Parvovirus virions are 23–28 nanometers (nm) in diameter and consist of the genome enclosed inside a capsid that is icosahedral in shape with a rugged surface.
The capsid is composed of 60 structurally equivalent polypeptide chains derived from the C-terminal end of a VP protein's sequence, interlocking extensively to form an icosahedron with 60 asymmetric, superficial triangular units.
Each of these cylinders potentially contains an opening to connect the exterior of the capsid to the interior, which mediates entry and exit of the genome.
[1][2] Each VP monomer contains a core beta-barrel structure called the jelly roll motif of eight strands arranged in two adjacent antiparallel beta sheets, labeled CHEF and BIDG after the individual strands, the latter forming the interior surface of the capsid.
In endosomes, many parvoviruses undergo a change in conformation so that the phospholipase A2 (PLA2) domain on the VP1 N-termini are exposed so the virion can penetrate lipid bilayer membranes.
[3][7][9] Messenger RNA (mRNA) that encodes NS1 is then transcribed from the genome by the DNA polymerase, capped and polyadenylated, and translated by host ribosomes to synthesize NS1.
[16] The back and forth, end-to-end pattern of rolling hairpin replication produces a concatemer containing multiple copies of the genome.
[17] Based on phylogenetic analysis of the SF3 helicase, parvoviruses split into two branches early in their evolutionary history, one of which contains viruses assigned to the subfamily Hamaparvovirinae.
[2] Telomere sequences have significant complexity and diversity, suggesting that many species have co-opted them to perform additional functions.
Species are grouped together in a genus based on phylogeny of the NS1 and SF3 helicase domains, as well as similarity of NS1 sequence identity and coverage.
B19 infection is often asymptomatic but can manifest in a variety of ways, including Fifth disease with its characteristic rash in children, persistent anemia in immunocompromised persons and in people who have underlying hemoglobinopathies,[20] transient aplastic crises, hydrops fetalis in pregnant women, and arthropathy.
[2] Canine parvovirus causes severe illness in dogs, the most common symptom being hemorrhagic enteritis, with up to a 70% mortality rate in pups but usually less than 1% in adults.
[21] Feline parvovirus, a closely related virus,[22] likewise causes severe illness in cats along with panleukopenia.
[23][24] In pigs, porcine parvovirus is a major cause of infertility as infection frequently leads to death of the fetus.
[25] Adeno-associated viruses have become an important vector for gene therapy aimed at treating genetic diseases, such as those caused by a single mutation.
rAAV essentially acts as a container that can traverse the cell membrane and deliver its nucleic acid cargo to the nucleus.
[26][27] Parvoviruses were discovered relatively late in comparison to other prominent virus families, potentially due to their small size.
In the late 1950s[28] and 1960s,[29] a variety of animal parvoviruses were discovered, including minute virus of mice,[30] which has since been used extensively to study rolling hairpin replication.
Over time, improvements in aspects such as vector design led to certain AAV gene therapy products reaching clinical efficacy in 2008 and being approved in the following years.