Passivation (chemistry)
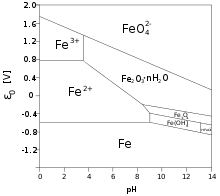
Boundaries between micro grains, if the oxide layer is crystalline, form an important pathway for oxygen to reach the unoxidized metal below.
Some compounds, dissolved in solutions (chromates, molybdates) form non-reactive and low solubility films on metal surfaces.
[18] Chromate conversion is a common way of passivating not only aluminium, but also zinc, cadmium, copper, silver, magnesium, and tin alloys.
As the uncoated surface is water-soluble, a preferred method is to form manganese or zinc compounds by a process commonly known as parkerizing or phosphate conversion.
Older, less effective but chemically similar electrochemical conversion coatings included black oxidizing, historically known as bluing or browning.
Some grades of stainless steel are especially resistant to rouging; parts made from them may therefore forgo any passivation step, depending on engineering decisions.
[23] Common among all of the different specifications and types are the following steps: Prior to passivation, the object must be cleaned of any contaminants and generally must undergo a validating test to prove that the surface is 'clean.'
The object is then placed in an acidic passivating bath that meets the temperature and chemical requirements of the method and type specified between customer and vendor.
After passivation, the parts are neutralized using a bath of aqueous sodium hydroxide, then rinsed with clean water and dried.
The passive surface is validated using humidity, elevated temperature, a rusting agent (salt spray), or some combination of the three.
These industry standards generally list several passivation processes that can be used, with the choice of specific method left to the customer and vendor.
Sodium dichromate is often required as an additive to oxidise the chromium in certain 'types' of nitric-based acid baths, however this chemical is highly toxic.
With citric acid, simply rinsing and drying the part and allowing the air to oxidise it, or in some cases the application of other chemicals, is used to perform the passivation of the surface.
It is not uncommon for some aerospace manufacturers to have additional guidelines and regulations when passivating their products that exceed the national standard.
The most common methods for validating the passivity of a part is some combination of high humidity and heat for a period of time, intended to induce rusting.
In the area of microelectronics and photovoltaic solar cells, surface passivation is usually implemented by thermal oxidation at about 1000 °C to form a coating of silicon dioxide.
A small molecule with the function of passivation is some kind of square that can be inserted where there is an empty space and then a complete layer is obtained.
These molecules will generally have lone electron pairs or pi-electrons, so they can bind to the defective states on the surface of the cell film and thus achieve passivation of the material.