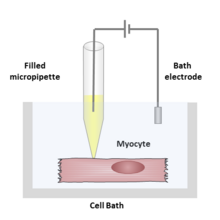
Patch clamp
In this case, the current passing across the membrane is controlled by the experimenter and the resulting changes in voltage are recorded, generally in the form of action potentials.
[2] This small size is used to enclose a cell membrane surface area or "patch" that often contains just one or a few ion channel molecules.
In some experiments, the micropipette tip is heated in a microforge to produce a smooth surface that assists in forming a high resistance seal with the cell membrane.
[3] The high resistance of this seal makes it possible to isolate electronically the currents measured across the membrane patch with little competing noise, as well as providing some mechanical stability to the recording.
Accurate tissue sectioning with compresstome vibratome or microtomes is essential, in addition to patch clamp methods.
Cell-attached and both excised patch techniques are used to study the behavior of individual ion channels in the section of membrane attached to the electrode.
This allows the recording of currents through single, or a few, ion channels contained in the patch of membrane captured by the pipette.
However, voltage-gated ion channels can be clamped successively at different membrane potentials in a single patch.
This results in channel activation as a function of voltage, and a complete I-V (current-voltage) curve can be established in only one patch.
The outer face of the vesicle must then be broken open to enter into inside-out mode; this may be done by briefly taking the membrane through the bath solution/air interface, by exposure to a low Ca2+ solution, or by momentarily making contact with a droplet of paraffin or a piece of cured silicone polymer.
[13] Outside-out patching gives the experimenter the opportunity to examine the properties of an ion channel when it is isolated from the cell and exposed successively to different solutions on the extracellular surface of the membrane.
The experimenter can perfuse the same patch with a variety of solutions in a relatively short amount of time, and if the channel is activated by a neurotransmitter or drug from the extracellular face, a dose-response curve can then be obtained.
[14] This ability to measure current through exactly the same piece of membrane in different solutions is the distinct advantage of the outside-out patch relative to the cell-attached method.
The longer formation process involves more steps that could fail and results in a lower frequency of usable patches.
The main difference lies in the fact that when the experimenter forms the gigaohm seal, suction is not used to rupture the patch membrane.
The perforated patch can be likened to a screen door that only allows the exchange of certain molecules from the pipette solution to the cytoplasm of the cell.
[15] Disadvantages include a higher access resistance, relative to whole-cell, due to the partial membrane occupying the tip of the electrode.
It can also take a significant amount of time for the antibiotic to perforate the membrane (about 15 minutes for amphothericin-B, and even longer for gramicidin and nystatin).
This technique was used as early as the year 1961, as described in a paper by Strickholm on the impedance of a muscle cell's surface,[16] but received little attention until being brought up again and given a name by Almers, Stanfield, and Stühmer in 1982,[17] after patch clamp had been established as a major tool of electrophysiology.
This leakage can be partially corrected for, however, which offers the opportunity to compare and contrast recordings made from different areas on the cell of interest.
[18] A combination of cellular imaging, RNA sequencing and patch clamp this method is used to fully characterize neurons across multiple modalities.
Combining classical classification methods with single cell RNA-sequencing post-hoc has proved to be difficult and slow.
It currently suffers from low throughput relative to other sequencing methods mainly due to the manual labor involved in achieving a successful patch-clamp recording on a neuron.
[20] Automated patch clamp systems have been developed in order to collect large amounts of data inexpensively in a shorter period of time.