Automated patch clamp
This work has been ongoing since the late 1990s by research labs and companies trying to reduce its complexity and cost of patch clamping manually.
The automation techniques try to reduce user error and variability in obtaining quality electrophysiology recordings from single cells.
The traditional manual method to patch clamp using glass pipettes was developed by Erwin Neher and Bert Sakmann and required a highly skilled technician.
The technician is essentially performing a balancing act trying to watch and manipulate several systems simultaneously (motion, pressure, and electrical signals).
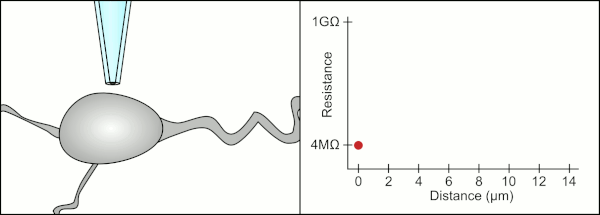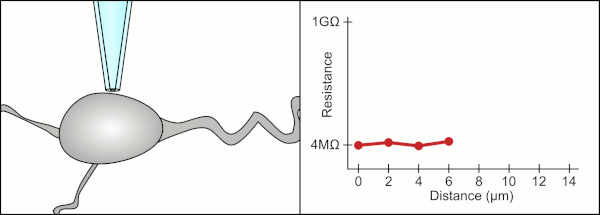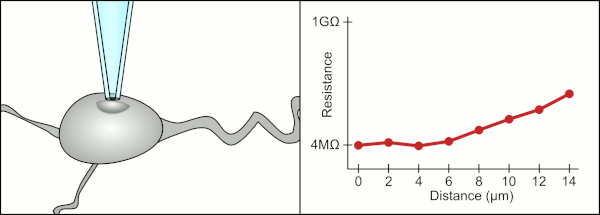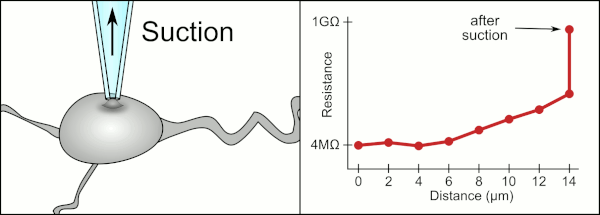
This environment also contains blood vessels, dendrites, axons, and glial cells which make it harder to form a gigaseal by clogging the 1-2μm diameter pipette tip.
Cells in vitro can be suspended in a fluid, made to adhere to a culture dish, or remain part of a piece of tissue that has been removed from the animal.
In the case of cells in suspension, the pipette is completely replaced with a microchip with holes that can create gigaseals and measure the electrical activity.
One system uses a traditional pipette and cells in a droplet suspension culture to obtain patch clamp recordings (see figure).
This has the added benefit of using traditional pipette fabrication systems that heat a glass capillary and pull it lengthwise to create the tapered tip used in patch clamping.
More common automation systems for suspensions cultures use microchips with tiny (1-2μm) holes in a planar substrate instead of pipettes to create the gigaseal and record from single cells.
This allows researchers to study ion channel behavior in more controlled environments without currents from other cells interfering, as usually occurs in neural networks.
[9] Neurons derived from stem cells cultured adherently can be lifted into suspension and have been successfully used on planar patch clamp devices.
One uses a patch chip, like those discussed above, along with surface treatments that cause the cultured cells to migrate to the orifices where the gigaseal is formed as they grow.
[12] By allowing the neurons to grow in culture, they form networks spontaneously, like those in the brain, which is more like the natural tissues than isolated cells in suspension.