Adenosine deaminase
[6] In the monomer form, the enzyme is a polypeptide chain,[7] folded into eight strands of parallel α/β barrels, which surround a central deep pocket that is the active site.
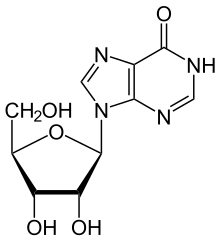
Inosine can then be deribosylated (removed from ribose) by another enzyme called purine nucleoside phosphorylase (PNP), converting it to hypoxanthine.
The reaction is stereospecific due to the location of the zinc, Asp295, and His238 residues, which all face the B-side of the purine ring of the substrate.
[8] The enzyme has been found in bacteria, plants, invertebrates, vertebrates, and mammals, with high conservation of amino acid sequence.
[6] The high degree of amino acid sequence conservation suggests the crucial nature of ADA in the purine salvage pathway.
[15] Deficient levels of ADA have also been associated with pulmonary inflammation, thymic cell death, and defective T-cell receptor signaling.
ADA2 is the predominant form present in human blood plasma and is increased in many diseases, particularly those associated with the immune system: for example rheumatoid arthritis, psoriasis, and sarcoidosis.
[citation needed] Total plasma ADA can be measured using high performance liquid chromatography or enzymatic or colorimetric techniques.
After incubation of plasma with a buffered solution of adenosine the ammonia is reacted with a Berthelot reagent to form a blue colour which is proportionate to the amount of enzyme activity.
[25] Cladribine and Pentostatin are anti-neoplastic agents used in the treatment of hairy cell leukemia; their mechanism of action is inhibition of adenosine deaminase.