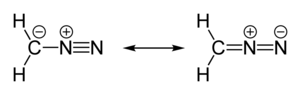
Diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894.
In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether.
The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions.
[8][9] Diazomethane reacts with alcohols or phenols in presence of boron trifluoride (BF3) to give methyl ethers.
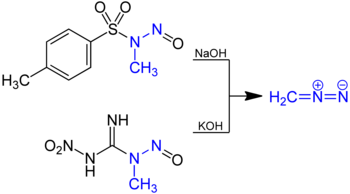
Diazomethane is prepared by hydrolysis of an ethereal solution of these N-methyl nitrosamides with aqueous base.
Examples include: Diazomethane reacts with alkaline solutions of D2O to give the deuterated derivative CD2N2.
Symptoms include chest discomfort, headache, weakness and, in severe cases, collapse.
In one instance a laboratory worker consumed a hamburger near a fumehood where he was generating a large quantity of diazomethane, and died four days later from fulminating pneumonia.
The compound explodes when heated beyond 100 °C, exposed to intense light, alkali metals, or calcium sulfate.