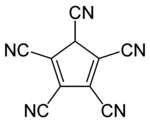
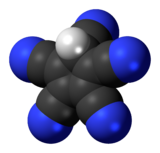
Pentacyanocyclopentadiene
Pentacyanocyclopentadiene is a derivative of cyclopentadiene with five cyano groups with the molecular formula C5H(CN)5.
[3][4] By virtue of a combination of mesomeric and aromatic stabilization of its anion, pentacyanocyclopentadiene is a superacid, with an estimated aqueous pKa of −11.
[5] The free acid was prepared by Reed in 2004 and was assigned a polymeric structure with protons that bridge planar C5(CN)5 units.
[6] Pentacyanocyclopentadiene is synthesised by coupling carbon disulfide and sodium cyanide in dimethylformamide before oxidation using ammonium persulfate and final purification generates the ammonium pentacyanocyclopentadiene salt.
Further reaction with sodium hydride generates NaC5(CN)5 which is a starting point for its coordination chemistry with transition metals.