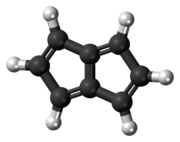
Pentalene
Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings.
[6] It is prepared from reaction of dihydropentalene (pyrolysis of an isomer of dicyclopentadiene) with n-butyllithium in solution and forms a stable salt.
In accordance with its structure proton NMR shows 2 signals in a 2 to 1 ratio.
The addition of two electrons removes the antiaromaticity; it becomes a planar 10π-electron aromatic species and is thus a bicyclic analogue of the cyclooctatetraene (COT) dianion C8H2−8.
The dianion can also be considered as two fused cyclopentadienyl rings, and has been used as a ligand in organometallic chemistry to stabilise many types of mono- and bimetallic complexes, including those containing multiple metal-metal bonds, and anti-bimetallics with extremely high levels of electronic communication between the centers.