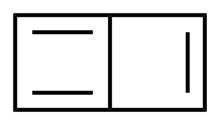
Butalene
Butalene is a polycyclic hydrocarbon composed of two fused cyclobutadiene rings.
[1] A reported possible synthesis of it involves an elimination reaction from a Dewar benzene derivative.
The structure itself can be envisioned as benzene with an internal bridge, and calculations indicate it is somewhat less stable than the open 1,4-didehydrobenzene biradical, the valence isomer in which that bridged bond is broken.
Thus, the most significant π bonding interactions involve conjugation around the periphery of the whole six-atom structure, similar to benzene, rather than cross-ring resonance along the bridging bond.
[2] Significant resonance around one or the other four-membered ring alone would be a less-stable antiaromatic form, as is seen in cyclobutadiene itself.