Peptidoglycan
Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like layer (sacculus) that surrounds the bacterial cytoplasmic membrane.
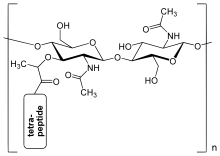
[1] The sugar component consists of alternating residues of β-(1,4) linked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM).
[4] Depending on pH growth conditions, the peptidoglycan forms around 40 to 90% of the cell wall's dry weight of gram-positive bacteria but only around 10% of gram-negative strains.
[8] The peptidoglycan layer within the bacterial cell wall is a crystal lattice structure formed from linear chains of two alternating amino sugars, namely N-acetylglucosamine (GlcNAc or NAG) and N-acetylmuramic acid (MurNAc or NAM).
L-form bacteria and mycoplasmas, both lacking peptidoglycan cell walls, do not proliferate by binary fission, but by a budding mechanism.
[15][16] In the course of early evolution, the successive development of boundaries (membranes, walls) protecting first structures of life against their environment must have been essential for the formation of the first cells (cellularisation).
[25][26] The aim of these modifications is to alter the properties of the cell wall, which plays a vital role in pathogenesis.
[25] Peptidoglycans can be degraded by several enzymes (lysozyme, glucosaminidase, endopeptidase...[25]), producing immunostimulatory fragments (sometimes called muropeptides[27]) that are critical for mediating host-pathogen interactions.
[28] Intracellular bacterial pathogens invade eukaryotic cells (which may lead to the formation of phagolysosomes and/or autophagy activation), or bacteria may be engulfed by phagocytes (macrophages, monocytes, neutrophils...).
The bacteria-containing phagosome may then fuse with endosomes and lysosomes, leading to degradation of bacteria and generation of polymeric peptidoglycan fragments and muropeptides.
[27] Mammals produce four secreted soluble peptidoglycan recognition proteins (PGLYRP-1, PGLYRP-2, PGLYRP-3 and PGLYRP-4) that recognize muramyl pentapeptide or tetrapeptide.
[28] After recognition of peptidoglycan, PGLYRPs activate polyphenol oxidase (PPO) molecules, Toll, or immune deficiency (IMD) signalling pathways.
[25][27] It is proposed, that the function of PGLYRP-2 is to prevent over-activation of the immune system and inflammation-induced tissue damage in response to NOD2 ligands (see below), as these muropeptides can no longer be recognized by NOD2 upon separation of the peptide component from MurNAc.
[27] Growing evidence suggests that peptidoglycan recognition protein family members play a dominant role in the tolerance of intestinal epithelial cells toward the commensal microbiota.
[28] Recently, it has been discovered, that PGLYRPs (and also NOD-like receptors and peptidoglycan transporters) are highly expressed in the developing mouse brain.
The NOD1 receptor is activated after iE-DAP (γ-d-glutamyl-meso-diaminopimelic acid) binding, while NOD2 recognizes MDP (muramyl dipeptide), by their LRR domains.
[27] In macrophages, N-acetylglucosamine generated by peptidoglycan degradation was found to inhibit hexokinase activity and induce its release from the mitochondrial membrane.
[28][26] C-type lectins are a diverse superfamily of mainly Ca2+-dependent proteins that bind a variety of carbohydrates (including the glycan skeleton of peptidoglycan), and function as innate immune receptors.
[32] But this TLR2-inducing activity could be due to cell wall lipoproteins and lipoteichoic acids that commonly co-purify with peptidoglycan.
[25][27] Peptidoglycan is immunologically active, which can stimulate immune cells to increase the expression of cytokines and enhance antibody-dependent specific response when combined with vaccine or as adjuvant alone.
[33] Some antibacterial drugs such as penicillin interfere with the production of peptidoglycan by binding to bacterial enzymes known as penicillin-binding proteins or DD-transpeptidases.
[36] Lysozyme, which is found in tears and constitutes part of the body's innate immune system exerts its antibacterial effect by breaking the β-(1,4)-glycosidic bonds in peptidoglycan (see above).