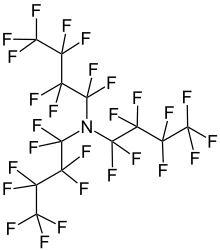
Perfluorotributylamine
Perfluorotributylamine (PFTBA), also referred to as FC43, is an organic compound with the chemical formula N(CF2CF2CF2CF3)3.
The high degree of fluorination significantly reduces the basicity of the central amine due to electron-withdrawing effects.
[1] It is prepared by electrofluorination of tributylamine using hydrogen fluoride as solvent and source of fluorine:[2] The compound has two commercial uses.
This application exploits the high solubility of oxygen and carbon dioxide in the solvent, as well as the low viscosity and toxicity.
Fluorofluids are generally of very low toxicity, so much that they have been evaluated as synthetic blood.