Periodic trends
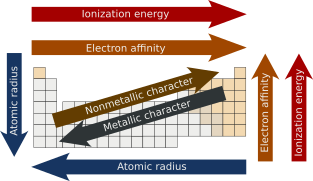
Major periodic trends include atomic radius, ionization energy, electron affinity, electronegativity, nucleophilicity, electrophilicity, valency, nuclear charge, and metallic character.
[3] Mendeleev's discovery of this trend allowed him to predict the existence and properties of three unknown elements, which were later discovered by other chemists and named gallium, scandium, and germanium.
In general, the atomic radius decreases as we move from left-to-right in a period, and it increases when we go down a group.
When we move down the group, the atomic radius increases due to the addition of a new shell.
But, from top-to-bottom of a group, as the number of shells increases, the effective nuclear charge will decrease.
The decrease in the atomic size results in a more potent force of attraction between the electrons and the nucleus.
In that case, the ionization energy decreases as atomic size increases due to adding a valence shell, thereby diminishing the nucleus's attraction to electrons.
[14] Trend-wise, as one progresses from left to right across a period, the electron affinity will increase as the nuclear charge increases and the atomic size decreases resulting in a more potent force of attraction of the nucleus and the added electron.
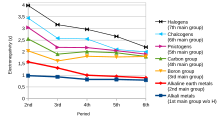
[15] The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity.
In simple terms, it is the measure of the combining capacity of an element to form chemical compounds.
[21][22] Trend-wise, while moving from left to right across a period, the number of valence electrons of elements increases and varies between one and eight.
Across each period, from left to right, the increasing attraction between the nuclei and the outermost electrons causes the metallic character to decrease.