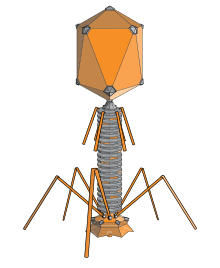
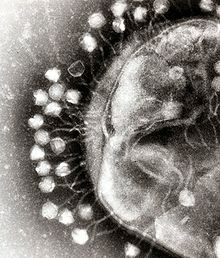
Bacteriophage
[4] Viruses are the most abundant biological entity in the water column of the world's oceans, and the second largest component of biomass after prokaryotes,[5] where up to 9x108 virions per millilitre have been found in microbial mats at the surface,[6] and up to 70% of marine bacteria may be infected by bacteriophages.
In 1896, Ernest Hanbury Hankin reported that something in the waters of the Ganges and Yamuna rivers in India had a marked antibacterial action against cholera and it could pass through a very fine porcelain filter.
[20] In 1915, British bacteriologist Frederick Twort, superintendent of the Brown Institution of London, discovered a small agent that infected and killed bacteria.
Independently, French-Canadian microbiologist Félix d'Hérelle, working at the Pasteur Institute in Paris, announced on 3 September 1917 that he had discovered "an invisible, antagonistic microbe of the dysentery bacillus".
For d'Hérelle, there was no question as to the nature of his discovery: "In a flash I had understood: what caused my clear spots was in fact an invisible microbe... a virus parasitic on bacteria.
[25] In 1969, Max Delbrück, Alfred Hershey, and Salvador Luria were awarded the Nobel Prize in Physiology or Medicine for their discoveries of the replication of viruses and their genetic structure.
Delbrück and Luria carried out the Luria–Delbrück experiment which demonstrated statistically that mutations in bacteria occur randomly and thus follow Darwinian rather than Lamarckian principles.
Phages were discovered to be antibacterial agents and were used in the former Soviet Republic of Georgia (pioneered there by Giorgi Eliava with help from the co-discoverer of bacteriophages, Félix d'Hérelle) during the 1920s and 1930s for treating bacterial infections.
D'Herelle "quickly learned that bacteriophages are found wherever bacteria thrive: in sewers, in rivers that catch waste runoff from pipes, and in the stools of convalescent patients.
[28] However, they were abandoned for general use in the West for several reasons: The use of phages has continued since the end of the Cold War in Russia,[30] Georgia, and elsewhere in Central and Eastern Europe.
The first regulated, randomized, double-blind clinical trial was reported in the Journal of Wound Care in June 2009, which evaluated the safety and efficacy of a bacteriophage cocktail to treat infected venous ulcers of the leg in human patients.
[32] On the other hand, phages of Inoviridae have been shown to complicate biofilms involved in pneumonia and cystic fibrosis and to shelter the bacteria from drugs meant to eradicate disease, thus promoting persistent infection.
[35] In 2017, a 68-year-old diabetic patient with necrotizing pancreatitis complicated by a pseudocyst infected with MDR A. baumannii strains was being treated with a cocktail of Azithromycin, Rifampicin, and Colistin for 4 months without results and overall rapidly declining health.
Because discussion had begun of the clinical futility of further treatment, an Emergency Investigational New Drug (eIND) was filed as a last effort to at the very least gain valuable medical data from the situation, and approved, so he was subjected to phage therapy using a percutaneously (PC) injected cocktail containing nine different phages that had been identified as effective against the primary infection strain by rapid isolation and testing techniques (a process which took under a day).
This proved effective for a very brief period, although the patient remained unresponsive and his health continued to worsen; soon isolates of a strain of A. baumannii were being collected from drainage of the cyst that showed resistance to this cocktail, and a second cocktail which was tested to be effective against this new strain was added, this time by intravenous (IV) injection as it had become clear that the infection was more pervasive than originally thought.
[36] Once on the combination of the IV and PC therapy the patient's downward clinical trajectory reversed, and within two days he had awoken from his coma and become responsive.
The original three-antibiotic cocktail was replaced by minocycline after the bacterial strain was found not to be resistant to this and he rapidly regained full lucidity, although he was not discharged from the hospital until roughly 145 days after phage therapy began.
In that same year, the FDA approved LISTEX (developed and produced by Micreos) using bacteriophages on cheese to kill Listeria monocytogenes bacteria, in order to give them generally recognized as safe (GRAS) status.
Due to their shared structural and biological characteristics, coliphages can serve as proxies for viral fecal contamination and the presence of pathogenic viruses such as rotavirus, norovirus, and HAV.
[44] Government agencies in the West have for several years been looking to Georgia and the former Soviet Union for help with exploiting phages for counteracting bioweapons and toxins, such as anthrax and botulism.
[45] Developments are continuing among research groups in the U.S. Other uses include spray application in horticulture for protecting plants and vegetable produce from decay and the spread of bacterial disease.
The bound, selected phages can be multiplied by reinfecting a susceptible bacterial strain, thus allowing them to retrieve the peptides encoded in them for further study.
[55] Sometimes prophages may provide benefits to the host bacterium while they are dormant by adding new functions to the bacterial genome, in a phenomenon called lysogenic conversion.
[59] To enter a host cell, bacteriophages bind to specific receptors on the surface of bacteria, including lipopolysaccharides, teichoic acids, proteins, or even flagella.
This specificity means a bacteriophage can infect only certain bacteria bearing receptors to which they can bind, which in turn, determines the phage's host range.
Polysaccharide-degrading enzymes are virion-associated proteins that enzymatically degrade the capsular outer layer of their hosts at the initial step of a tightly programmed phage infection process.
[60] As phage virions do not move independently, they must rely on random encounters with the correct receptors when in solution, such as blood, lymphatic circulation, irrigation, soil water, etc.
Early studies of bactioriophage T4 (1962-1964) provided an opportunity to gain understanding of virtually all of the genes that are essential for growth of the bacteriophage under laboratory conditions.
[77] Evolutionary mechanisms shaping the genomes of bacterial viruses vary between different families and depend upon the type of the nucleic acid, characteristics of the virion structure, as well as the mode of the viral life cycle.
The use of phages is preferred to the more conventional dye marker because they are significantly less absorbed when passing through ground waters and they are readily detected at very low concentrations.