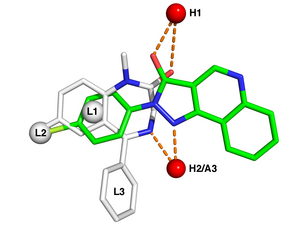
Pharmacophore
Furthermore, pharmacophore models can be used to identify through de novo design or virtual screening novel ligands that will bind to the same receptor.
Typical pharmacophore features include hydrophobic centroids, aromatic rings, hydrogen bond acceptors or donors, cations, and anions.
In modern computational chemistry, pharmacophores are used to define the essential features of one or more molecules with the same biological activity.
A database of diverse chemical compounds can then be searched for more molecules which share the same features arranged in the same relative orientation.
[4] Historically, the modern idea of pharmacophore was popularized by Lemont Kier, who mentions the concept in 1967[5] and uses the term in a publication in 1971.