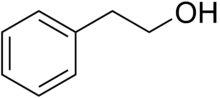
Phenethyl alcohol
Most common is the Friedel-Crafts reaction between benzene and ethylene oxide in the presence of aluminium trichloride.
The reaction affords the aluminium alkoxide that is subsequently hydrolyzed to the desired product.
[3] Phenethyl alcohol can also be prepared by the reaction between phenylmagnesium bromide and ethylene oxide: Phenethyl alcohol can also be produced by biotransformation from L-phenylalanine using immobilized yeast Saccharomyces cerevisiae.
[4] It is also possible to produce phenethyl alcohol by the reduction of phenylacetic acid using sodium borohydride and iodine in THF.
[5] Phenethyl alcohol is found in extract of rose, carnation, hyacinth, Aleppo pine, orange blossom, ylang-ylang, geranium, neroli, and champaca.