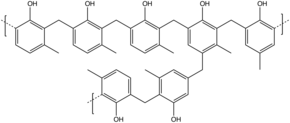
Phenol formaldehyde resin
[2][3] There are many variations in both production and input materials that are used to produce a wide variety of resins for special purposes.
In either case, the curing agent is a source of formaldehyde which provides bridges between novolac chains, eventually completely crosslinking the system.
Phenol, formaldehyde, water and catalyst are mixed in the desired amount, depending on the resin to be formed, and are then heated.
The first part of the reaction, at around 70 °C, forms a thick reddish-brown tacky material, which is rich in hydroxymethyl and benzylic ether groups.
Being thermosets, hydroxymethyl phenols will crosslink on heating to around 120 °C to form methylene and methyl ether bridges through the elimination of water molecules.
The high crosslinking gives this type of phenolic resin its hardness, good thermal stability, and chemical imperviousness.
Exterior plywood, oriented strand boards (OSB), engineered high-pressure laminate are typical applications.
The resin fully polymerizes (cures) during this process forming the thermoset polymer matrix.
Phenolic resin is used as a binder in loudspeaker driver suspension components which are made of cloth.
Higher end billiard balls are made from phenolic resins, as opposed to the polyesters used in less expensive sets.
[citation needed] Atmospheric re-entry spacecraft use phenol formaldehyde resin as a key component in ablative heat shields (e.g. AVCOAT on the Apollo modules).
This reaction absorbs significant thermal energy, insulating the deeper layers of the heat shield.