Phosphate
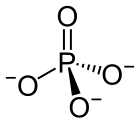
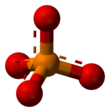
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid.
[2] The addition and removal of phosphate groups (phosphorylation and dephosphorylation) are key steps in cell metabolism.
In water solution, orthophosphoric acid and its three derived anions coexist according to the dissociation and recombination equilibria below[3] Values are at 25 °C and 0 ionic strength.
Around pH 4.7 (mid-way between the first two pKa values) the dihydrogen phosphate ion, [H2PO4]−, is practically the only species present.
Around pH 9.8 (mid-way between the second and third pKa values) the monohydrogen phosphate ion, [HPO4]2−, is the only species present.
This means that salts of the mono- and di-phosphate ions can be selectively crystallised from aqueous solution by setting the pH value to either 4.7 or 9.8.
The various metaphosphate ions (which are usually long linear polymers) have an empirical formula of (PO3)− and are found in many compounds.
[5] Inorganic phosphate is generally denoted Pi and at physiological (homeostatic) pH primarily consists of a mixture of [HPO4]2− and [H2PO4]− ions.
Free orthophosphate anions can be released by the hydrolysis of the phosphoanhydride bonds in ATP or ADP.
An important occurrence of phosphates in biological systems is as the structural material of bone and teeth.
The hard dense enamel of mammalian teeth may contain fluoroapatite, a hydroxy calcium phosphate where some of the hydroxyl groups have been replaced by fluoride ions.
[6] For patients who are unable to get enough phosphorus in their daily diet, phosphates are used as dietary supplements, usually because of certain disorders or diseases.
Rock phosphate can also be found in Egypt, Israel, Palestine, Western Sahara, Navassa Island, Tunisia, Togo, and Jordan, countries that have large phosphate-mining industries.
Phosphorite mines are primarily found in: In 2007, at the current rate of consumption, the supply of phosphorus was estimated to run out in 345 years.
[11] Phosphorus comprises 0.1% by mass of the average rock[12] (while, for perspective, its typical concentration in vegetation is 0.03% to 0.2%),[13] and consequently there are quadrillions of tons of phosphorus in Earth's 3×1019-ton crust,[14] albeit at predominantly lower concentration than the deposits counted as reserves, which are inventoried and cheaper to extract.
Some phosphate rock deposits, such as Mulberry in Florida,[15] are notable for their inclusion of significant quantities of radioactive uranium isotopes.
This is a concern because radioactivity can be released into surface waters[16] from application of the resulting phosphate fertilizer.
[21] The three principal phosphate producer countries (China, Morocco and the United States) account for about 70% of world production.
In ecological terms, because of its important role in biological systems, phosphate is a highly sought after resource.
Addition of high levels of phosphate to environments and to micro-environments in which it is typically rare can have significant ecological consequences.
In the context of pollution, phosphates are one component of total dissolved solids, a major indicator of water quality, but not all phosphorus is in a molecular form that algae can break down and consume.
Mining operations processing phosphate rock can leave tailings piles containing elevated levels of cadmium, lead, nickel, copper, chromium, and uranium.
Unless carefully managed, these waste products can leach heavy metals into groundwater or nearby estuaries.
Uptake of these substances by plants and marine life can lead to concentration of toxic heavy metals in food products.