Phosphofructokinase 1
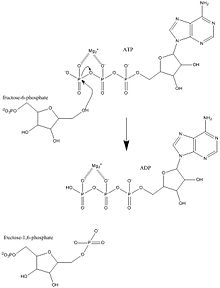
Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis.
The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.
Mammalian PFK1 is a 340kd[3] tetramer composed of different combinations of three types of subunits: muscle (M), liver (L), and platelet (P).
Tissue-specific changes in PFK activity and isoenzymic content contribute significantly to the diversities of glycolytic and gluconeogenic rates which have been observed for different tissues.
The N-terminal domain has a catalytic role binding the ATP, and the C-terminal has a regulatory role [7] PFK1 is an allosteric enzyme whose activity can be described using the symmetry model of allosterism[8] whereby there is a concerted transition from an enzymatically inactive T-state to the active R-state.
Thus a graph plotting PFK1 activity against increasing F6P concentrations would adopt the sigmoidal curve shape traditionally associated with allosteric enzymes.
[9] In B. stearothermophilus PFK1, the positively charged side chain of Arg162 residue forms a hydrogen-bonded salt bridge with the negatively charged phosphate group of F6P, an interaction which stabilizes the R state relative to the T state and is partly responsible for the homotropic effect of F6P binding.
This swap in positions between adjacent amino acid residues inhibits the ability of F6P to bind the enzyme.
Similarly, inhibitors such as ATP and PEP bind to the same allosteric site and facilitate the formation of the T state, thereby inhibiting enzyme activity.
ATP concentration build up indicates an excess of energy and does have an allosteric modulation site on PFK1 where it decreases the affinity of PFK1 for its substrate.
Because PFK regulates glycolytic flux, serotonin plays a regulatory role in glycolysis [13] There are three phosphofructokinase genes in humans: A genetic mutation in the PFKM gene results in Tarui's disease, which is a glycogen storage disease where the ability of certain cell types to utilize carbohydrates as a source of energy is impaired.
[14] Tarui disease is a glycogen storage disease with symptoms including muscle weakness (myopathy) and exercise induced cramping and spasms, myoglobinuria (presence of myoglobin in urine, indicating muscle destruction) and compensated hemolysis.
[17][18] When cancer cells grow and divide quickly, they initially do not have as much blood supply, and can thus have hypoxia (oxygen deprivation), and this triggers O-GlcNAcylation at serine 529 of PFK.
This may be due to redirecting glucose flux towards the pentose phosphate pathway to generate NADPH to detoxify reactive oxygen species.
[19] Herpes simplex type 1 and phosphofructokinase: Some viruses, including HIV, HCMV and Mayaro affect cellular metabolic pathways such as glycolysis by a MOI-dependent increase in the activity of PFK.