Phosphoglucomutase
If the cell is low on energy, then glucose 6-phosphate will travel down the glycolytic pathway, eventually yielding two molecules of adenosine triphosphate.
If the cell is in need of biosynthetic intermediates, glucose 6-phosphate will enter the pentose phosphate pathway, where it will undergo a series of reactions to yield riboses and/or NADPH, depending on cellular conditions.
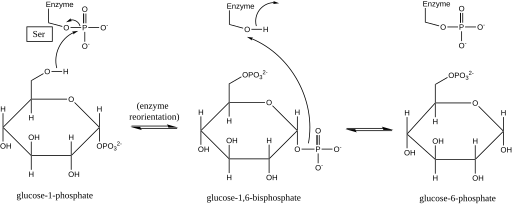
[4][5] Later structural studies confirmed that the single site in the enzyme that becomes phosphorylated and dephosphorylated is the oxygen of the active-site serine residue (see diagram below).
[6][7] A bivalent metal ion, usually magnesium or cadmium, is required for enzymatic activity and has been shown to complex directly with the phosphoryl group esterified to the active-site serine.
[10] Each phosphoglucomutase monomer can be divided into four sequence domains, I-IV, based on the enzyme’s default spatial configuration (see image at right).
[11] The burial of the active site (including Ser-116, the critical residue on the enzyme that is phosphorylated and dephosphorylated) in the hydrophobic interior of the enzyme serves to exclude water from counterproductively hydrolyzing critical phosphoester bonds while still allowing the substrate to access the active site.
[16][21] Although the phenotype and severity of the disease is highly variable, common symptoms include: exercise intolerance, exercise-induced hyperammonemia, abnormal glycogen accumulation in muscle biopsy, elevated serum CK, abnormal serum transferrin (loss of complete N-glycans), short stature, cleft palate, bifid uvula, and hepatopathy.
Under such circumstances, the heart rate and breathing increases inappropriately given the exercise intensity, in an attempt to maximize the delivery of oxygen and blood borne fuels to the muscle cell.
Free fatty acids are the slowest of the body's bioenergetic systems to produce ATP by oxidative phosphorylation, at approximately 10 minutes.
[25][26] These studies supported that when the exercise is stopped or sufficient ATP is produced from other fuels (such as free fatty acids), then the ATP reservoir normalizes and the buildup of AMP and other nucleotides covert into nucleosides and leave the muscle cell to be converted into uric acid, known as myogenic hyperuricemia.
Unfortunately, the studies on PGM1-CDG only tested for serum ammonia and lactate, so it is currently unknown definitively whether PGM1-CDG individuals also experience myogenic hyperuricemia.