Phycoerythrin
[3][7] Phycobiliproteins have many practical application to them including imperative properties like hepato-protective, anti-oxidants, anti-inflammatory and anti-aging activity of PBPs enable their use in food, cosmetics, pharmaceutical and biomedical industries.
The light energy is captured by phycoerythrin and is then passed on to the reaction centre chlorophyll pair, most of the time via the phycobiliproteins phycocyanin and allophycocyanin.
B-phycoerythrin (B-PE) and R-phycoerythrin (R-PE) from red algae in addition to α and β chains have a third, γ subunit contributing both linker and light-harvesting functions, because it bears chromophores.
These γ chains from the Protein Data Bank are very small and consist only of three or six recognizable amino acids [15][16], whereas described at the beginning of this section linker γ chain is large (for example 277 amino acid long 33 kDa in case of γ33 from red algae Aglaothamnion neglectum) [17][2].
This is because the electron density of the gamma-polypeptide is mostly averaged out by its threefold crystallographic symmetry and only a few amino acids can be modeled [15][16][18][19].
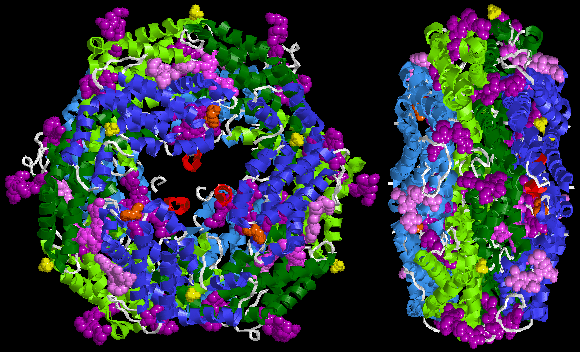
Below is sample crystal structure of R-phycoerythrin from Protein Data Bank: Absorption peaks in the visible light spectrum are measured at 495 and 545/566 nm, depending on the chromophores bound and the considered organism.
This could be a result of the depth in the water column that a specific alga typically resides and a consequent need for greater or less efficiency of the accessory pigments.
With advances in imaging and detection technology which can avoid rapid photobleaching, protein fluorophores have become a viable and powerful tool for researchers in fields such as microscopy, microarray analysis and Western blotting.
Even a small increase in fluorescent efficiency could reduce background noise and lower the rate of false-negative results.
R-Phycoerythrin (also known as PE or R-PE) is useful in the laboratory as a fluorescence-based indicator for the presence of cyanobacteria and for labeling antibodies, most often for flow cytometry.


4 .