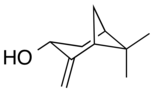
Pinocarveol
It is a bicyclic monoterpenoid, which is a combination of two isoprene units with one hydroxyl group as a substituent.
[1] It exists as either trans- or cis-pinocarveol, referring to stereochemical orientation of the oxygen as compared to the methylene bridge.
It is a naturally occurring molecule in numerous plant species including Eucalyptus globulus and Picea abies.
[3][4] Pinocarveol can be synthesized by heating a mixture of turpentine, selenium dioxide, and hydrogen peroxide.
The selenium dioxide acts as a catalyst while the hydrogen peroxide oxidizes the pinene found in turpentine.