Polarimeter
is a physical property and defined as the optical rotation α at a path length l of 1 dm, a concentration c of 10 g/L, a temperature T (usually 20 °C) and a light wavelength λ (usually sodium D line at 589.3 nm):[4] This tells us how much the plane of polarization is rotated when the ray of light passes through a specific amount of optically active molecules of a sample.
Therefore, the optical rotation depends on temperature, concentration, wavelength, path length, and the substancebeing analyzed.
When the analyzer is also placed in a similar position it allows the light waves coming from the polarizer to pass through it.
H is a half shade device which divides the field of polarized light emerging out of the Nicol P into two halves, generally of unequal brightness.
The rotation of the analyzer can be measured with the help of a scale C. Working principle: To understand the need of a half-shade device, let us suppose that it is not present.
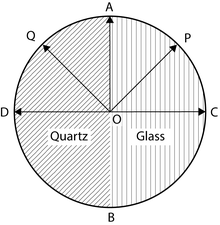
Thus components OA and OD will combine to form a resultant vibration along OQ which makes the same angle with optic axis as OP.
Now if the Principal plane of the analyzing Nicol is parallel to OP then the light will pass through the glass half unobstructed.
Determination of specific rotation: In order to determine a specific rotation of an optically active substance (say, sugar), the polarimeter tube is first filled with pure water and the analyzer is adjusted for equal darkness (both the halves should be equally dark) point.
Now the polarimeter tube is filled with a sugar solution of known concentration and again the analyzer is adjusted in such a way that again the equally dark point is achieved.
The detector was positioned at the opposite end of a tube containing the optically active sample, and the user used his/her eye to judge the "alignment" when least light was observed.
Although most manual polarimeters produced today still adopt this basic principle, the many developments applied to the original opto-mechanical design over the years have significantly improved measurement performance.
The introduction of a half-wave plate increased "distinction sensitivity", whilst a precision glass scale with vernier drum facilitated the final reading to within ca.
Most modern manual polarimeters also incorporate a long-life yellow LED in place of the more costly sodium arc lamp as a light source.
Fully automatic polarimeters are now widely used and simply require the user to press a button and wait for a digital readout.
Fast automatic digital polarimeters yield an accurate result within a few seconds, regardless of the rotation angle of the sample.
In addition, they provide continuous measurement, facilitating High-performance liquid chromatography and other kinetic investigations.
Special techniques as temperature controlled sample tubes reduce measuring errors and ease operation.
The angle of rotation of an optically active substance can be affected by: Most modern polarimeters have methods for compensating or/and controlling these errors.
Traditionally, a sucrose solution with a defined concentration was used to calibrate polarimeters relating the amount of sugar molecules to the light polarization rotation.
A more reliable and stable standard was found: crystalline quartz which is oriented and cut in a way that it matches the optical rotation of a normal sugar solution, but without showing the disadvantages mentioned above.
In order to ensure reliable and comparable results, quartz plates can be calibrated and certified by metrology institutes.
Alternatively, calibration may be checked using a Polarization Reference Standard, which consists of a plate of quartz mounted in a holder perpendicular to the light path.
These standards are available, traceable to NIST, by contacting Rudolph Research Analytical, located at 55 Newburgh Road, Hackettstown, NJ 07840, USA.
[11] Because many optically active chemicals such as tartaric acid, are stereoisomers, a polarimeter can be used to identify which isomer is present in a sample – if it rotates polarized light to the left, it is a levo-isomer, and to the right, a dextro-isomer.
With a known concentration of a sample, polarimetry may also be applied to determine the specific rotation (a physical property) when characterizing a new substance.
Concentration and purity measurements are especially important to determine product or ingredient quality in the food & beverage and pharmaceutical industries.
Often, the sugar refineries use a modified polarimeter with a flow cell (and used in conjunction with a refractometer) called a saccharimeter.

1. Light source
2. Unpolarized light
3. Linear polarizer
4. Linearly polarized light
5. Sample tube containing chiral molecules under study
6. Optical rotation due to molecules
7. Rotatable linear analyzer
8. Detector