Polyaspartic acid
[6] The first isolation of synthetic oligomeric sodium polyaspartate, obtained by thermal polycondensation of aspartic acid, was reported by Hugo Schiff in late 19th century.
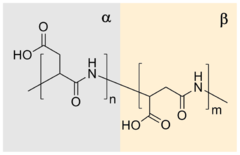
[5] In contrast, the repeating unit of synthetic polyaspartic acid may exist in four isomeric forms, depending on the stereochemistry of starting material (D- and L-aspartic acid) and synthetic procedure leading to α and β links.
In a subsequent step the resulting polysuccinimide is treated with aqueous sodium hydroxide, which yields partial opening of the succinimide rings.
Main benefits from their application is increasing of the conversion rate and higher molecular weight of the product.
[14][15] Polyaspartic acid can also be synthesized by polymerization of maleic anhydride in presence of ammonium hydroxide.
In one procedure, aspartic acid polymerizes at 180 °C concomitant with dehydration and the formation of a poly(succinimide).
[2] As it can be synthesized in an environmentally friendly way and is biodegradable, polyaspartate is a potential green alternative to several materials such as sodium polyacrylate used in disposable diapers and agriculture.
[26] The level of water uptake which is inversely related to the mechanical properties of the hydrogel can be tuned by changing the crosslinking density.