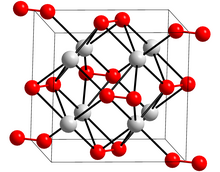
Potassium peroxide
Potassium peroxide is an inorganic compound with the molecular formula K2O2.
Potassium peroxide reacts with water to form potassium hydroxide and oxygen: Potassium peroxide is a highly reactive, oxidizing white to yellowish solid which, while not flammable itself, reacts violently with flammable materials.
It decomposes violently on contact with water.
[1] The standard enthalpy of formation of potassium peroxide is ΔH f 0 = −496 kJ/mol.
Potassium peroxide is used as an oxidizing agent and bleach (due to the peroxide), and to purify air.