Polanyi potential theory
[1] Afterwards, he published a fully developed paper in 1916, which included experimental verification by his students and other authors.
Einstein wrote back to Bredig stating: The papers of your M. Polanyi please me a lot.
I have checked over the essentials in them and found them fundamentally correct.Polanyi later described this event by saying: Bang!
Polanyi's model of adsorption was met with much criticism for several decades after publication years.
His simplistic model for determining adsorption was formed during the time of the discovery of Peter Debye's fixed dipoles, Niels Bohr's atomic model, and well as the developing theory of intermolecular forces and electrostatic forces by key figures in the chemistry world including W.H.
However, Polanyi was not able to participate in many of these discussions because he served as a medical officer for the Austro-Hungarian army in the Serbian front during World War I. Polanyi wrote about this experience saying: I myself was protected for a while against any knowledge of these developments by serving as a medical officer in the Austro-Hungarian Army from August 1914 to October 1918, and by the subsequent revolutions and counter revolutions that lasted until the end of 1919.
Members of less-well-informed circles elsewhere continued to be impressed for some time by the simplicity of my theory and its wide experimental verifications.
[1]Polanyi described that the “turning point” of the acceptance of his model of adsorption occurred when Fritz Haber asked him to defend his theory in full in the Kaiser Wilhelm Institute for Physical Chemistry in Berlin, Germany.
Many key players in the scientific world were present in this meeting including Einstein.
Years later, Polanyi described his ordeal by concluding, Professionally, I survived the occasion only by the skin of my teeth.Polanyi continued to provide supporting evidence in proving the validity of his model years after this meeting.
[1] Polanyi's 'deliverance' (as he described it) from these rejections and criticism of his model occurred in 1930, when Fritz London proposed a new theory of cohesive forces founded on the theories of quantum mechanics on the polarization of electronic systems.
Polanyi wrote to London asking, “Are these forces subject to screening by intervening molecules?
Would a solid acting by these forces possess a spatially fixed adsorption potential?” After computational analysis, a joint publication was made between Polanyi and London claiming that the adsorptive forces behaved similarly to the model that Polanyi had proposed.
Other research have been performed loosely involving the potential theory of Polanyi such as the capillary condensation phenomenon discovered by Richard Adolf Zsigmondy.
His research proved that condensation of vapors can occur in narrow pores below the standard saturated vapour pressure.
[4] This model is applicable in the case of gases at a surface at constant temperature.
, is equal to the chemical potential of the gas at an infinitely large distance from the surface,
Since the temperature remains constant, the difference in chemical potential formula can be integrated over pressures
is Considering that the partial pressure of the gases relates to the concentration, the adsorption potential,
The potential theory underwent many refinements and changes throughout the years since its first report.
is fit to experimental data can be simplified to Other studies have used the Dubinin–Astakhov in a similar form of
[10] Later on, experiments also showed that it can model ionic polycyclic aromatic hydrocarbons such as phenols and anilines.
Historically, the theory was used to model nonuniform adsorbates and multi-components solutes.
In the study done by Yang and Xing,[6] the theory have been shown to better fit the adsorption isotherm than Langmuir, Freundlich, and partition.
According to the Polyani theory the surface defect curvatures of carbon nanoparticles could affect their adsorption.
Using this theory, researchers are hoping to be able to design carbon nanoparticles for specific needs such as using them as sorbents in environmental studies.
In one of the earlier studies conducted by Manes, M., & Hofer, L. J. E.,[11] the Polyani theory was used to characterize liquid-phase adsorption isotherms on various concentrations activated carbon using a wide range of organic solvent.
Because of the results, the study introduced the possibility of predicting isotherms for similar systems using minimal data.
The researchers who conducted the experiment speculate that steric effects of carbon tetrachloride and cyclohexane may have played a role.
In the experiment conducted by Rosene and Manes,[12] the competitive adsorption of glucose, urea, benzoic acid, phthalide, and p-nitrophenol.
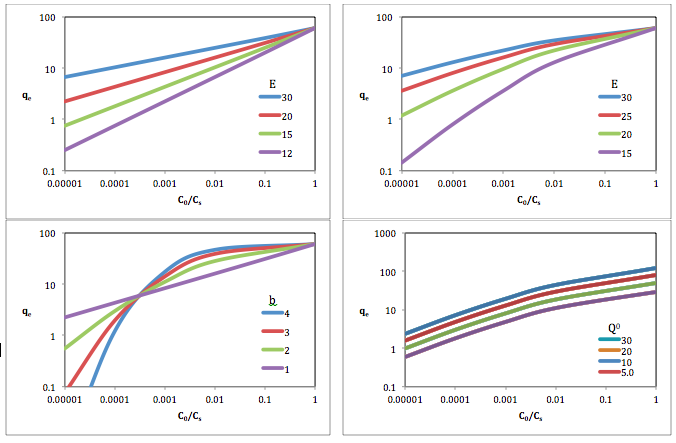
Top-left: Q 0 = 60; b = 1
Top-right: Q 0 = 60; b = 1.5
Bottom-left: Q 0 = 60; E = 20
Bottom-right: E = 20; b = 1.5