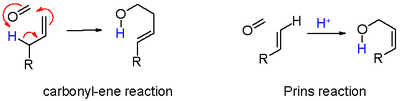
Prins reaction
The original reactants employed by Dutch chemist Hendrik Jacobus Prins [de] in his 1919 publication were styrene (scheme 2), pinene, camphene, eugenol, isosafrole and anethole.
As a consequence, commercial availability of lower olefin coupled with an aldehyde produced from oxidation of low boiling paraffin increased the curiosity to study the olefin-aldehyde condensation.
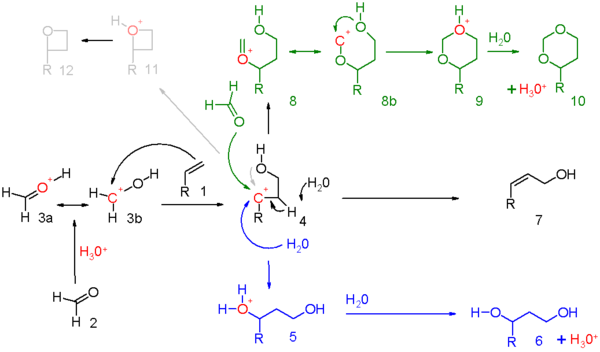
The carbonyl reactant (2) is protonated by a protic acid and for the resulting oxonium ion 3 two resonance structures can be drawn.
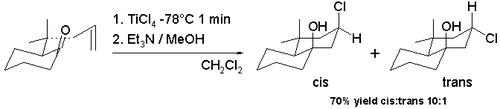
[7] This observed cis diastereoselectivity is due to the intermediate formation of a trichlorotitanium alkoxide making possible an easy delivery of chlorine to the carbocation ion from the same face.
The trans isomer is preferred (98% cis) when the switch is made to a tin tetrachloride reaction at room temperature.
With lewis acid stannic chloride the oxonium ion is activated and the pinacol rearrangement of the resulting Prins intermediate results in ring contraction and referral of the positive charge to the TIPS ether which eventually forms an aldehyde group in the final product as a mixture of cis and trans isomers with modest diastereoselectivity.